Coordination Entity and Sphere play an invaluable role. These complex frameworks help streamline operations, foster collaboration among various components within an enterprise and facilitate communication among them. We will explore all levels of coordination entities and spheres by looking into their characteristics, uses and applications.
What is Coordination Entity?
A coordination entity, also known as a coordination complex, refers to a molecule or ion formed by the combination of a central metal atom or ion and surrounding molecules or ions, called ligands. The central metal atom/ion is typically a transition metal, and the ligands are molecules or ions that have one or more pairs of electrons available for bonding to the metal center.
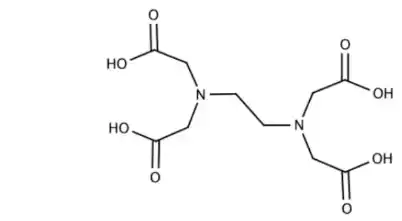
In a coordination entity, the ligands attach to the central metal atom/ion through coordination bonds, which are often dative or coordinate covalent bonds. These bonds involve the sharing of electron pairs between the metal and the ligand, resulting in the formation of a stable complex.
Key characteristics of coordination entities include:
- Central Metal Atom/Ion: The central metal atom/ion plays a pivotal role in the coordination complex. Its properties, including oxidation state and electron configuration, influence the type of ligands that can bind to it and the overall stability of the complex.
- Ligands: Ligands are molecules or ions that surround the central metal atom/ion. They can be monodentate (binding through a single atom), bidentate (binding through two atoms), or even polydentate (binding through multiple atoms). Ligands are responsible for determining the coordination number and geometry of the complex.
- Coordination Number: The coordination number refers to the number of ligands attached to the central metal atom/ion. It determines the overall geometry of the complex and influences its chemical and physical properties.
- Coordination Geometry: The arrangement of ligands around the central metal atom/ion determines the coordination geometry of the complex. Common geometries include linear, square planar, tetrahedral, and octahedral.
- Stability and Properties: The formation of coordination entities can lead to enhanced stability and unique chemical and physical properties compared to the individual components. Coordination complexes often exhibit distinct colors, magnetic properties, and reactivity patterns.
- Nomenclature: Coordination compounds have a specific nomenclature system that describes the arrangement of ligands around the central metal atom/ion. This system includes the names of the ligands, the central metal’s name along with its oxidation state, and additional prefixes and suffixes.
Coordination entities are widely studied in coordination chemistry, which explores the interactions between metal ions and ligands. They have numerous practical applications, ranging from catalysis and medicine to materials science and environmental studies.
What is Coordination Sphere?
The term “coordination sphere” refers to the central metal atom or ion along with its surrounding ligands in a coordination complex. It represents the immediate environment around the central metal in the complex and includes the ligands directly bonded to the metal through coordination bonds.
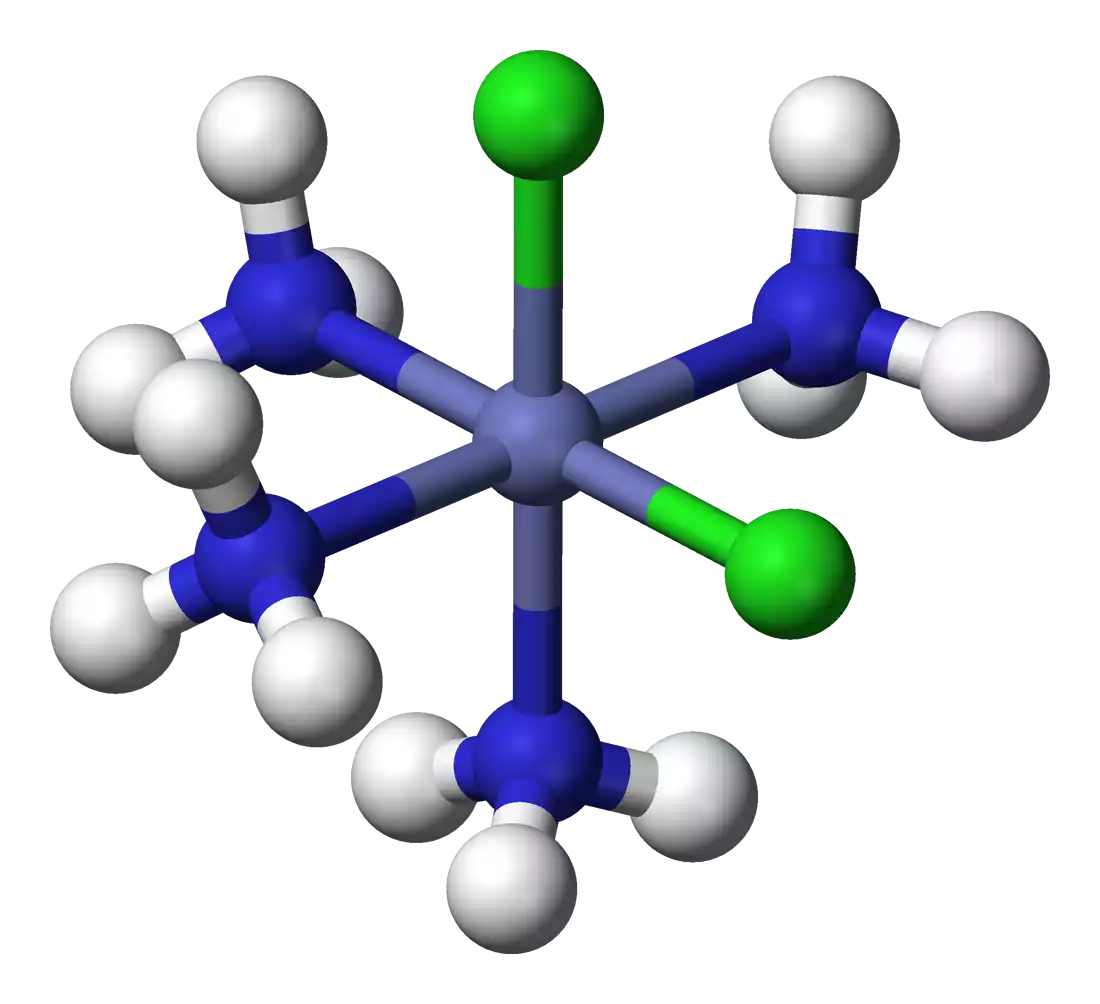
In a coordination complex, the coordination sphere can be visualized as a molecular entity that encompasses the central metal atom or ion and the ligands attached to it. The coordination sphere is often enclosed within square brackets “[ ]” and is followed by the charge of the entire complex.
For example, in the coordination complex [Cu(NH3)4(H2O)2]2+, the coordination sphere is “[Cu(NH3)4(H2O)2]2+”. Here, the coordination sphere consists of the central copper (Cu) ion and its surrounding ligands, which are four ammonia (NH3) molecules and two water (H2O) molecules.
The concept of the coordination sphere is essential in describing the structure, geometry, and properties of coordination complexes. It helps chemists understand the arrangement of ligands around the central metal, which influences the complex’s physical and chemical characteristics. The coordination sphere is a fundamental aspect of coordination chemistry and is used in nomenclature and notation to represent the overall complex.
Differences Between Coordination Entity and Sphere
Coordination entities and coordination spheres are related concepts in coordination chemistry, but they refer to different aspects of coordination complexes.
Here are the key differences between coordination entities and coordination spheres:
- Definition:
- Coordination Entity: A coordination entity refers to the entire complex formed by the central metal atom/ion and its surrounding ligands, including all the atoms and ions involved in the complex.
- Coordination Sphere: The coordination sphere specifically refers to the central metal atom/ion and the ligands directly bonded to it through coordination bonds. It represents the immediate environment around the central metal.
- Composition:
- Coordination Entity: It includes the central metal atom/ion, ligands, and any counterions or additional species present in the complex.
- Coordination Sphere: It includes only the central metal atom/ion and the ligands directly coordinated to it.
- Representation:
- Coordination Entity: It is typically represented by a molecular formula that includes all the components of the complex, along with their stoichiometry.
- Coordination Sphere: It is often enclosed within square brackets “[ ]” and represents the central metal atom/ion and its directly coordinated ligands, usually followed by the charge of the entire complex.
- Focus:
- Coordination Entity: It emphasizes the overall composition, stoichiometry, and structure of the entire coordination complex.
- Coordination Sphere: It focuses specifically on the interactions and arrangement of ligands around the central metal atom/ion.
- Usage:
- Coordination Entity: The term “coordination entity” is used in a broader context to refer to the complete complex, including the coordination sphere.
- Coordination Sphere: The term “coordination sphere” is used to describe the central metal and its directly coordinated ligands.
- Nomenclature:
- Coordination Entity: Nomenclature of coordination entities involves naming both the central metal and the ligands, along with any additional information about the complex.
- Coordination Sphere: The coordination sphere is often part of the nomenclature, enclosed within square brackets, and is used to represent the central metal and directly coordinated ligands.
While coordination entities encompass the entire composition of a coordination complex, including ligands, central metal, and other components, the coordination sphere specifically refers to the central metal and its directly bonded ligands. The coordination sphere is a subset of the coordination entity and provides crucial information about the immediate environment around the central metal atom/ion.
Representation and Visualization
Representation and visualization are crucial aspects when discussing coordination entities and coordination spheres in coordination chemistry. They help in understanding the structural arrangement of ligands and the central metal atom/ion within a complex.
Here’s how representation and visualization differ for coordination entities and coordination spheres:
Representation and Visualization of Coordination Entities:
- Structural Formulas: Coordination entities are often represented using structural formulas, which depict the connectivity between the central metal atom/ion and the surrounding ligands. Bonds are typically shown using lines or dashes, with different symbols or colors representing the elements involved.
- Ball-and-Stick Models: Physical models or computer-generated images known as ball-and-stick models provide a three-dimensional representation of coordination entities. These models use balls to represent atoms (metal and ligands) and sticks to represent bonds between them.
- Space-Filling Models: Space-filling models illustrate the relative sizes of atoms and molecules in a coordination entity. Atoms are represented as spheres that fill the available space, giving a better sense of the spatial arrangement of the complex.
- Lewis Structures: Lewis structures are simplified representations that show only the valence electrons and bond connections between the central metal and ligands.
Representation and Visualization of Coordination Spheres:
- Square Brackets Notation: Coordination spheres are often enclosed within square brackets “[ ]” and placed at the beginning of the chemical formula of the entire complex. This notation clearly delineates the central metal and its directly coordinated ligands from other components.
- Coordination Geometry Diagrams: Diagrams may be used to visualize the coordination geometry of the ligands around the central metal ion. These diagrams provide a simplified depiction of the complex’s spatial arrangement without showing the actual atoms and bonds.
- VSEPR (Valence Shell Electron Pair Repulsion) Models: VSEPR models predict the shapes of coordination spheres by considering the repulsion between electron pairs around the central metal ion. These models help visualize the arrangement of ligands without explicitly showing the atoms.
Representation and visualization techniques vary for coordination entities and coordination spheres. Coordination entities are typically depicted using structural formulas, ball-and-stick models, and space-filling models to illustrate the complete complex’s structure. On the other hand, coordination spheres are often denoted using square brackets and may involve diagrams or models that focus specifically on the ligand arrangement around the central metal.
Significance and Applications
The distinction between coordination entities and coordination spheres has significance and applications in various scientific fields. Understanding these concepts contributes to advancements in chemistry, materials science, biology, and even interdisciplinary studies.
Here’s an overview of their significance and applications:
Significance:
- Structural Understanding: Differentiating between coordination entities and coordination spheres enhances our understanding of the structural arrangement of ligands and central metal ions within a complex. This knowledge is fundamental for predicting and explaining the properties and reactivity of coordination compounds.
- Nomenclature and Communication: Clear differentiation allows chemists to communicate complex information about coordination compounds more effectively through standardized nomenclature. It ensures accurate representation and description of complex structures.
- Interdisciplinary Connections: Coordination chemistry bridges various scientific disciplines, such as biology, medicine, catalysis, and materials science. Proper understanding of coordination entities and coordination spheres enables researchers to apply concepts across these disciplines.
Applications:
- Catalysis: Coordination complexes are often used as catalysts in chemical reactions. Understanding the coordination sphere’s geometry and ligand properties is crucial for designing efficient catalysts for industrial processes.
- Medicine and Pharmaceuticals: Coordination compounds play a role in medicinal chemistry, where metal-based drugs are developed for treating diseases such as cancer. A thorough understanding of coordination entities aids in designing effective drug candidates.
- Materials Science: Coordination compounds are used to create novel materials with specific properties, such as magnetic, electrical, or optical behavior. By controlling the coordination sphere, researchers can tailor material properties for various applications.
- Environmental Chemistry: Coordination complexes are involved in environmental processes, including metal ion transport, water purification, and pollutant removal. Knowledge of coordination entities and their behavior contributes to addressing environmental challenges.
- Bioinorganic Chemistry: Coordination compounds are present in biological systems, where they play essential roles in enzymes, biomolecules, and metal ion transport. Understanding the coordination sphere helps decipher the bioinorganic chemistry underlying biological processes.
- Cultural Heritage Preservation: Coordination chemistry is used to develop methods for preserving cultural heritage artifacts by understanding the interaction of metal ions with historical materials.
- Molecular Imaging: Coordination complexes are used as contrast agents in medical imaging techniques such as MRI (magnetic resonance imaging) to enhance image quality and provide diagnostic information.
- Photophysics and Photochemistry: Certain coordination compounds exhibit interesting photophysical and photochemical properties. Understanding the coordination sphere’s influence on these properties is vital for applications in sensors, LEDs, and photovoltaic devices.
- Education and Research: Proper comprehension of coordination entities and coordination spheres is essential for educating students in chemistry and related fields. Researchers rely on this knowledge to design and synthesize new compounds for various applications.
The significance of distinguishing between coordination entities and coordination spheres lies in their wide-ranging applications across scientific domains. This understanding enables researchers and professionals to harness the unique properties of coordination compounds for technological advancements and solving real-world challenges.
Conclusion
The differentiation between coordination entities and coordination spheres is a fundamental concept in coordination chemistry with far-reaching implications. Understanding these distinctions enhances our grasp of complex structures, facilitates effective communication, and paves the way for innovative applications in catalysis, medicine, materials science, and more.
This knowledge not only advances scientific research but also contributes to solving real-world challenges across diverse fields, showcasing the interconnectedness of these concepts in driving progress and discovery.