Strong and Weak Acids
An acid is any chemical compound or species which donates or accepts protons in reactions and accepts electron pairs in return. They can be further subdivided into strong acids and weak acids, with strong acids dissociating completely while weak ones partially dissolve in water-based solutions while the former only partially does so.
Why are Strong and Weak acids important in terms of the human body?
- Digestion: Strong acids like hydrochloric acid (HCl), found in our stomachs, play an indispensable part in aiding in our digestive process. They aid in breaking down food into its constituent parts while stimulating digestive enzymes to ensure efficient absorption of essential nutrients and avoid infections. Furthermore, strong acids also kill harmful bacteria to keep us healthier overall.
- pH Regulation: Both strong and weak acids, along with weak bases, help regulate pH levels in the body. Different organs and systems require particular pH ranges in order to function at their best – for instance, blood has a slightly alkaline pH maintained through its bicarbonate buffer system involving carbonic acid as an intermediate acid that balances out with bicarbonate ions to keep its slightly alkaline environment.
- Acid-base balance: Our bodies maintain an ideal acid-base equilibrium using complex mechanisms involving strong and weak acids and bases. Achieving such balance is necessary to support proper cell functioning, enzyme activity, and overall physiological processes – it plays an integral part of life itself! Various organs – like kidneys and lungs – play their part by working collaboratively in keeping levels of acids and bases within our systems under control.
- Buffering capacity: Weak acids and conjugate bases act as buffers, helping to regulate pH levels by absorbing or releasing hydrogen ions (H+). Buffers prevent sudden pH shifts which could harm cells or tissues by acting as absorbers of hydrogen ions absorbed from solution or released during changes. Buffering is essential in biological systems because it prevents drastic pH swings which could otherwise have severe detrimental consequences on cells and tissues.
- Cellular processes: Enzyme activity, membrane transport, and signal transduction all depend upon an ideal pH environment to function optimally. Both strong and weak acids play an integral part in maintaining this ideal pH environment for these processes to function smoothly.
- Acid-base disorders: Imbalances between acid-base levels can result in either acidosis or alkalosis, both with potentially severe health ramifications. Medical diagnosticians use strong and weak acids as tests of acidity-base status and as guides for developing treatment plans.
Definition of Strong Acids
Strong acids are those which dissociate completely into their constituent ions upon dissolving in water, dispersing into various constituent ions that make up molecules at high concentrations of hydronium ion ions (H3O+). Their high level of ionization causes constituent ions exposed to it to break apart completely upon exposure; ultimately leading to dissociation of individual elements and dissolution of molecules altogether.

Strong acids differ from less acidic ones by being capable of discharging large quantities of hydrogen ions in solution, creating strong acidity. Their pH values tend to drop, with conductivity tending to rise due to an ionic presence; their strong acidic nature is attributable to chemical structures that facilitate easy dissociation and ionization processes.
Strong acids include hydrochloric Acid (HCl), sulfuric Acid (H2SO4), and Nitric Acid (HNO3) hydrobromic Acids such as Hydrobromic acid HBr and Perchloric acid HClO4 when introduced into the water they dissociate completely, liberating anions as well as hydronium Ions as byproducts.
As an illustration, if an acid named H-A is considered, the separation of HA acid can be given as,
HA(aq) + H2O(l) à A–(aq) + H3O+(aq)
If the acid molecule contains many protons which are released, it is displayed as shown below. This example illustrates the dissociation process of diprotic acid. It is able to release two protons.
H2B(aq) + H2O(l) à B2-(aq) + H3O+(aq)
The pH of a solution is significantly affected by strong acids since strong acids emit H+ ions in the solution. The pH is determined by the amount of H+. The relation between the H+ concentration and pH could be described as follows.
pH = -log[H+(aq)]
The pH of a solution is significantly affected by strong acids since strong acids emit H+ ions in the solution. The pH is determined by the amount of H+. The relation between the H+ concentration and pH could be described as follows.
pH = -log[H+(aq)]
pH = -log[0.1 molL-1 ]
= 1
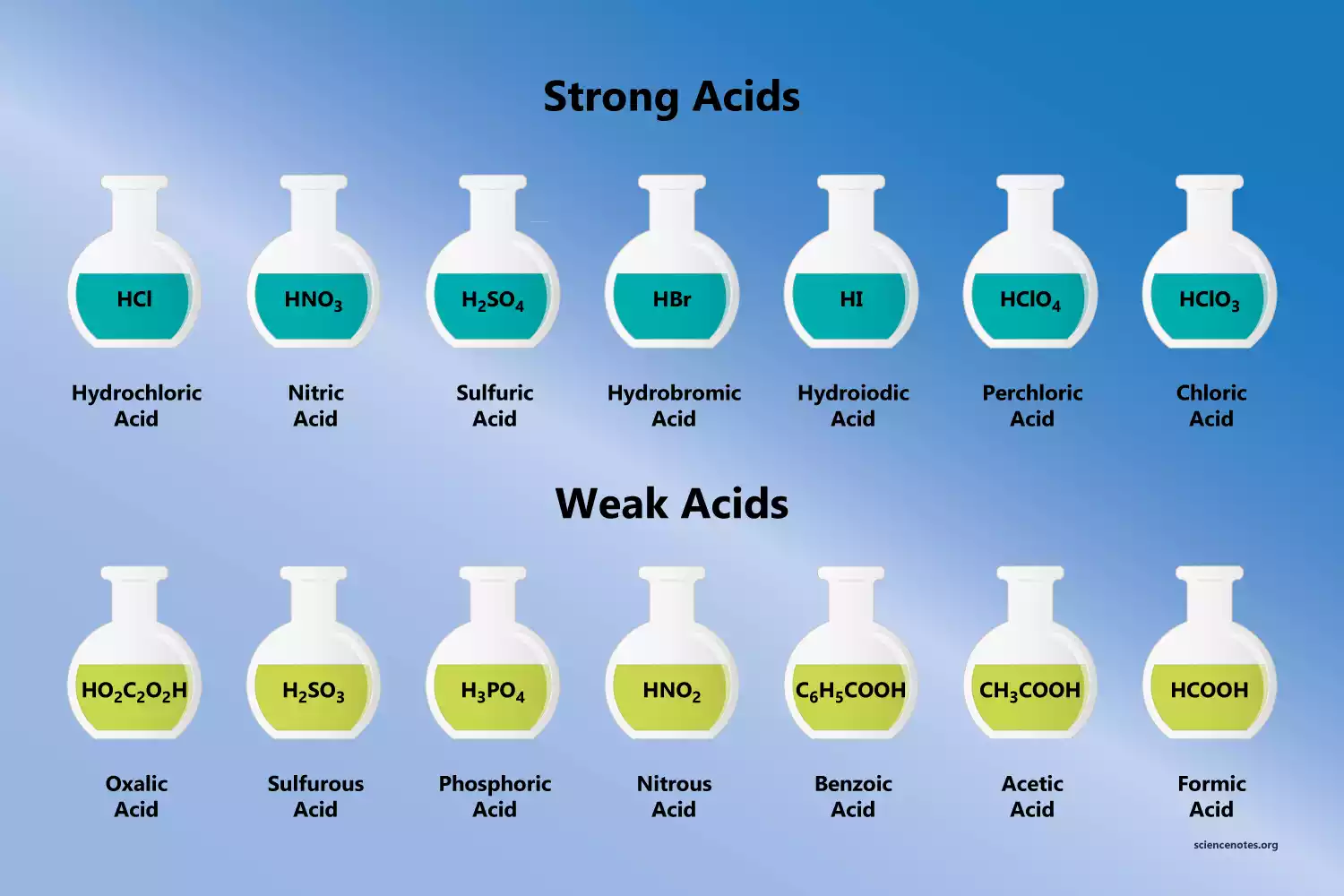
What are the 7 Strong acids?
- Sulfuric acid (H2SO4)
- Chloric acid (HClO3)
- Hydrochloric acid (HCl)
- Hydroiodic acid (HI)
- Nitric acid (HNO3)
- Perchloric acid (HClO4)
- Hydrobromic acid (HBr)
What are the uses of strong acids in daily life?
Cleaning and descaling acids such as hydrochloric acid (HCl), as well as sulfuric acid (H2SO4), are extensively utilized for cleaning and descaling reasons. They are effective in removing mineral deposits, rust, and scale from surfaces, equipment, and pipes.
- pH adjustment: Strong acids are employed to lower the pH of solutions or substances. Hydrochloric Acid is a solution to control the pH of swimming pools, water treatment facilities, and various industrial procedures when the requirement for pH-regulating conditions are required.
- Laboratory experiments: Strong acids are frequently used in laboratories for various experiments, such as titrations, chemical syntheses, and pH adjustments. They play a crucial role in the preparation of solutions and in analytical techniques.
- Battery electrolytes: Strong acids, particularly sulfuric acid, are used as electrolytes in lead-acid batteries commonly found in automobiles and backup power systems. The acid facilitates the electrochemical reactions within the battery, allowing for the storage and release of electrical energy.
- Metal etching: Strong acids are employed in metal etching processes. For example, nitric acid (HNO3) is used to etch designs or patterns onto metal surfaces, such as in jewelry making or circuit board manufacturing.
- pH control in swimming pools: Strong acids, such as hydrochloric acid, are utilized in swimming pools to lower the pH and maintain proper water chemistry. This helps prevent the growth of algae and harmful bacteria, ensuring safe and hygienic swimming conditions.
Definition of Weak Acids
A weak acid is one that dissociates in a limited way or transforms to form ions in its constituents when it is dissolved in water. Contrary to stronger acidic compounds, weak acids can not fully donate the proton (H+) to water molecules, leading to fewer ions of hydronium (H3O+). The dissociation that occurs in weak acids is caused by their less Ionization.
Weak acids have a less acidic pH than strong acids due to the fact that they release fewer hydrogen ions in the solution. They possess a greater level of pH and less conductivity when compared with strong acids because the amount of ions tend to be lower.
The weak acidic character of the compounds is due to a variety of factors, including their presence in functional groups which help stabilize undissociated acid molecules or impede the process of dissociation. These acids play a significant role in a variety of biological and chemical procedures because they play a role in reversible reactions as well as aid in pH regulation as well as a buffer system.
The general equation for the dissociation of a weak acid, represented as HA, can be written as follows:
HA ⇌ H+ + A-
The acid dissociation constant, Ka, is defined as the ratio of the concentrations of the products (H+ and A-) to the concentration of the undissociated acid (HA) at equilibrium. Mathematically, it can be expressed as:
Ka = [H+][A-] / [HA]
The acid dissociation constant is specific to each weak acid and is temperature-dependent. It provides information about the relative strength of the acid and its ability to donate protons in solution. A higher value of Ka indicates a stronger acid, while a lower value indicates a weaker acid.
Knowing Ka is essential to understanding weak acids; its value allows one to calculate various quantities that pertain to weak acid solutions such as degree of dissociation, pH value, and concentration of ions within solution solutions. The acid dissociation constant is an essential parameter in understanding the behavior of weak acids and their equilibrium reactions.
What are the 7 weak acids?
- Nitrous acid (HNO2)
- Formic acid (HCOOH)
- Sulfurous acid (H2SO3)
- Benzoic acid (C6H5COOH)
- Hydrofluoric acid (HF)
- Acetic acid (CH3COOH)
- Phosphoric acid (H3PO4)
What are the uses of weak acids in daily life?
- Food and drinks: Small amounts of weak acidic compounds like vinegar (acetic acid) and citric acid (found in citrus fruits), along with lactic acid found in yogurt serve to both preserve food and beverages as well as add flavorings into beverages and foods. They give a sweet or tart taste, and also in preventing the growth of specific microorganisms.
- Cleaning your home: Acids that are weak such as acetic acid are commonly used in cleaners. They’re effective at removing minerals, stains as well as soap scum, from a range of materials, such as glass bathrooms, countertops, and even bathroom fittings.
- Personal care items: Weak acid is used in many personal care products like shampoos, cleansers for the skin, and conditioners. They assist in changing the pH levels of items to be slightly acidic and are beneficial in maintaining healthy hair and skin.
- Cleaning and decaling common household items: weak acids, including citric acid as well as oxalic acid, are used for cleaning and descaling household products. They can be effective in eliminating mineral deposits off kettles, coffee makers showerheads, showerheads, and many various other areas.
- Treatment of water: weak acids, including weak organic acids, are utilized for water treatment. They aid in controlling the pH as well as alkalinity in water, thus preventing the corrosion of pipes, and also reducing the development of scale.
Comparison table of Strong Acids and Weak Acids
Here’s a comparison chart highlighting the key differences between strong and weak acids:
| Aspect | Strong Acids | Weak Acids |
|---|---|---|
| Degree of Dissociation | Completely dissociate in water | Partially dissociate in water |
| Ionization Constant (Ka) | Large Ka value, indicating a high degree of ionization | Small Ka value, indicating lower degree of ionization |
| Conductivity | Highly conductive due to complete dissociation | Relatively lower conductivity due to partial dissociation |
| pH Value | Low pH value (high acidity) | Higher pH value (lower acidity) |
| Reactivity | Highly reactive and corrosive | Comparatively less reactive |
| Examples | Hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3) | Acetic acid (CH3COOH), carbonic acid (H2CO3), formic acid (HCOOH) |
What are some similarities between strong and weak acids?
Although their characteristics differ greatly, strong acids and weak acids share certain similarities. First and foremost, all acid types exhibit acidic characteristics when mixed with water, giving off hydrogen ions (H+). This results in hydronium ions (H3O+). All acids share this fundamental acidic characteristic regardless of strength.
Strong acids and weak acids both contain hydrogen atoms that can be ionized; typically attached to electronegative elements like chlorine or oxygen, this allows both types of acids to participate in acid-base reactions that increase the acidity levels of solutions.
Additionally, both strong acids and weak acids are capable of engaging in chemical reactions, including neutralization processes that combine acids with bases to form salts and water; all acids share this ability for chemical transformations.
As is evident by their similar properties, strong acids, and weak acids differ significantly in terms of dissociation degree and behavior; strong acids completely ionize water while weak acids only partially do; these differences result in variations between their acidities, conductivities, and reactivities which ultimately determine acidity, conductivity and reactivity levels.
Conclusion
Strong acids and Weak acids differ by virtue of their degrees of dissociation, ionization constants, conductivity levels, pH values, reactivity levels, and examples. Strong acids dissociate in water completely while having high degrees of ionization with high conductivity levels that linger at low pH levels and highly reactive properties. Strong acids include hydrochloric and sulfuric acids.
Weak acids are able to only dissociate from water and with low levels of Ionization, a lower conductivity as well as higher pH which makes them less reactivity compared to stronger acids. Example of weak acids include acetic and carbonic acids.
Although different, strong and weak acids share similar acidic properties with one another as well as being involved in chemical reactions. Understanding their distinctions and similarities is imperative in many fields such as chemistry, biology and medicine.