Metals and Metalloids, which are both elements on the periodic chart, have unique properties and behaviors. Metalloids have properties in between metals and nonmetals. They are known for having a lustrous surface and high conductivity.
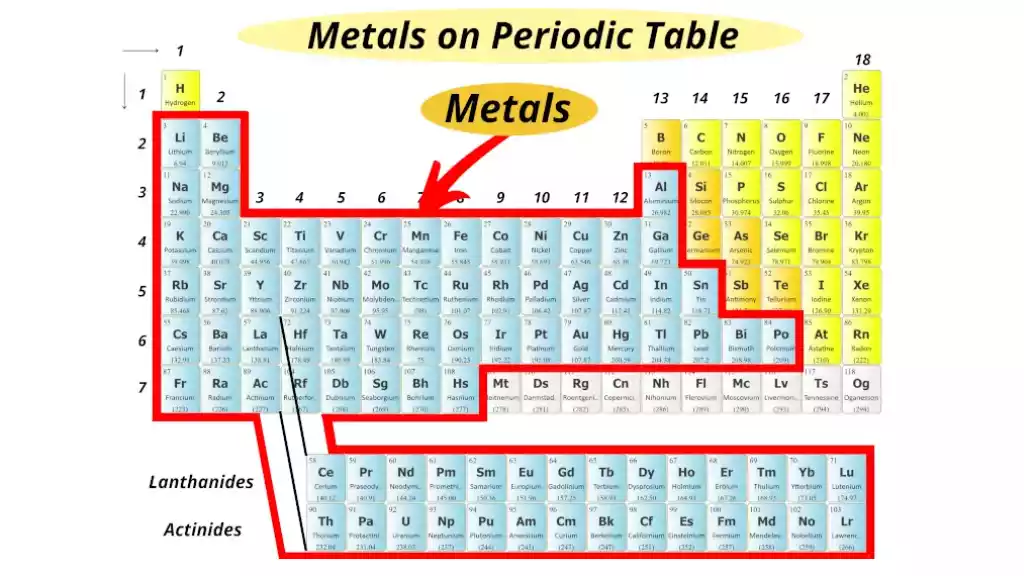
Definition of Metals
Metals are chemical elements characterized by their metallic appearance and characteristics such as high thermal and electrical conductivity, malleability, and ductility. Most metallic elements remain solid at room temperatures (excluding mercury) while possessing an appealing metallic sheen that gives off their characteristic metallic sheen.
Metals’ free electrons make them excellent conductors of electricity and heat, making them indispensable components in applications involving electrical wiring or power generation. This property makes metals indispensable.
Metals are distinguished by their malleability; that is, their ability to be formed into different shapes without shattering under pressure from hammering and pressing. Metals can thus be formed into various objects and structures such as tools and machinery without shattering in any form.
Metals are highly malleable, meaning they can be formed into thin sheets and wires without snapping, making them indispensable in the production and creation of thin metal sheets, such as those used in electrical systems. This property makes metals especially versatile as an essential building block of fabrication for thin sheets as well as wires used by such systems.
Metals possess low ionization energies, making it easier for their electrons to leave and produce positive ions – this property contributes to their high level of reactivity with other materials (particularly non-metals ).
Metals like iron, aluminum, and copper are examples of common types of metals; white gold, silver titanium as well as the physical form of gold itself may also qualify as metals. Metals have many applications across industries including construction, transportation, and manufacturing.
Properties of Metals
These properties include:
- Metallic Luster: Due to their ability to reflect light, metals have a characteristic shine on their surface. Metals have a distinctive appearance due to this property.
- High Electrical Conductivity: Metals have a high electrical conductivity. Metals have electrons that are free or delocalized and can freely move throughout their structure. This allows for an easy flow of electricity.
- High Thermal Conductivity: Metals are also efficient in conducting heat due to their high thermal conductivity. Metals are good at transferring heat, so they can be used in applications requiring efficient heat transfer.
- Malleability: Metals can be hammered or rolled into thin sheets, shapes, or rolls without breaking. This property is due to metallic bonding, where metal atoms casted past one another without affecting the overall structure.
- Ductility: Metals can be drawn in thin wires and not break. This property is a result of metallic bonding which allows metal atoms to be rearranged without structural damage.
- High Boiling and Melting Points: Metals have higher melting and boiling point than non-metals. Metals have strong bonds that require significant energy to break. This results in high temperatures to change the state of metals.
- Density: Metals have a large mass per volume because of the close packing of metal atoms into their crystal lattice structure.
- Reactivity: Metals have varying degrees of reactivity. Alkali meter some highly reactive metals that readily react with substances such as water or acids. Noble metals are less reactive than other metals and resistant to corrosion or oxidation.
Metals are used in many applications. These include construction, electrical wiring, and transportation. Metals are iron, copper, and aluminum. They also include gold, silver, and titanium.
Examples of Metals
Metals play an invaluable role both industrially and naturally.
Here are a few common metals.
- Iron (Fe): Iron is one of the most abundant metals, used widely across various industries and fields of study. Iron can be processed into steel that will in turn be utilized for construction projects, transportation systems, and producing various goods and materials.
- Aluminum (Al): Aluminum is an economical, corrosion-resistant metal widely utilized by aerospace, packaging, and transport industries.
- Copper (Cu): Copper is an outstanding conductor of electricity and heat, making it suitable for electrical wiring, plumbing fixtures, and electronic devices.
- Gold (Au): Gold is highly esteemed for its beauty, rarity, and value, making it popularly used for jewelry making, coin production, and as a status symbol; in electronics and dentistry settings alike it provides advantages that rival those found elsewhere.
- Silver (Ag): Another precious metal, is commonly used for jewelry, silverware, and photography applications as well as antimicrobial applications for medical uses, including wound dressings.
- Titanium (Ti): An extremely lightweight yet strong and corrosion-resistant metal, finds wide use across various industries including aerospace, medical implant manufacturing, and sporting equipment manufacturing.
- Zinc (Zn): Zinc has many uses in industry; most notably galvanization of other metals as an effective protective layer, battery manufacturing, and nutritional supplements being among them. It can also serve as an alloy component.
- Nickel (Ni): Nickel can be found in stainless steel products that offer corrosion and heat resistance, batteries, and magnets and is used as an energy storage source in batteries. Furthermore, nickel serves as an industrial catalyst in chemical reactions, batteries, and magnets – among other uses.
- Lead (Pb): Lead was once widely utilized in numerous applications such as pipes, batteries, and radiation shielding; however due to its toxic qualities its usage has significantly declined over the years.
- Mercury (Hg): Mercury is one of the few metals capable of existing in liquid form at room temperature and was commonly used in thermometers and barometers before becoming toxic enough for electrical switches – prompting its gradual decline over time.
Metals have many applications and industries they are used in, from automobile manufacturing to medical applications and aerospace development.
Definition of Metalloids
The metalloid group (commonly referred to as semi-metals and semiconductors) refers to elements with properties similar to both metals and non-metals; their location on a periodic chart places them between metals and non-metals.
Metalloids can be defined by several metallic properties, including moderate electrical conductivity and their ability to form alloys with other metals. Furthermore, metalloids also possess nonmetallic features like brittleness as well as covalent compounds with non-metals forming covalent compounds.
Metalloids conduct electricity differently depending on their environment. Under some conditions, metalloids may conduct as effectively as metals do while, in otconductivitytheir conductivity resembles that of non-metals.
Metalloids exhibit diverse chemical reactions. Mangermaniumds like germanium and silicon have found wide apsemiconductorhe semiconductor industry because of their ability to regulate electrical current flow; such elements play a critical role in creating electronic devices and computer chips.
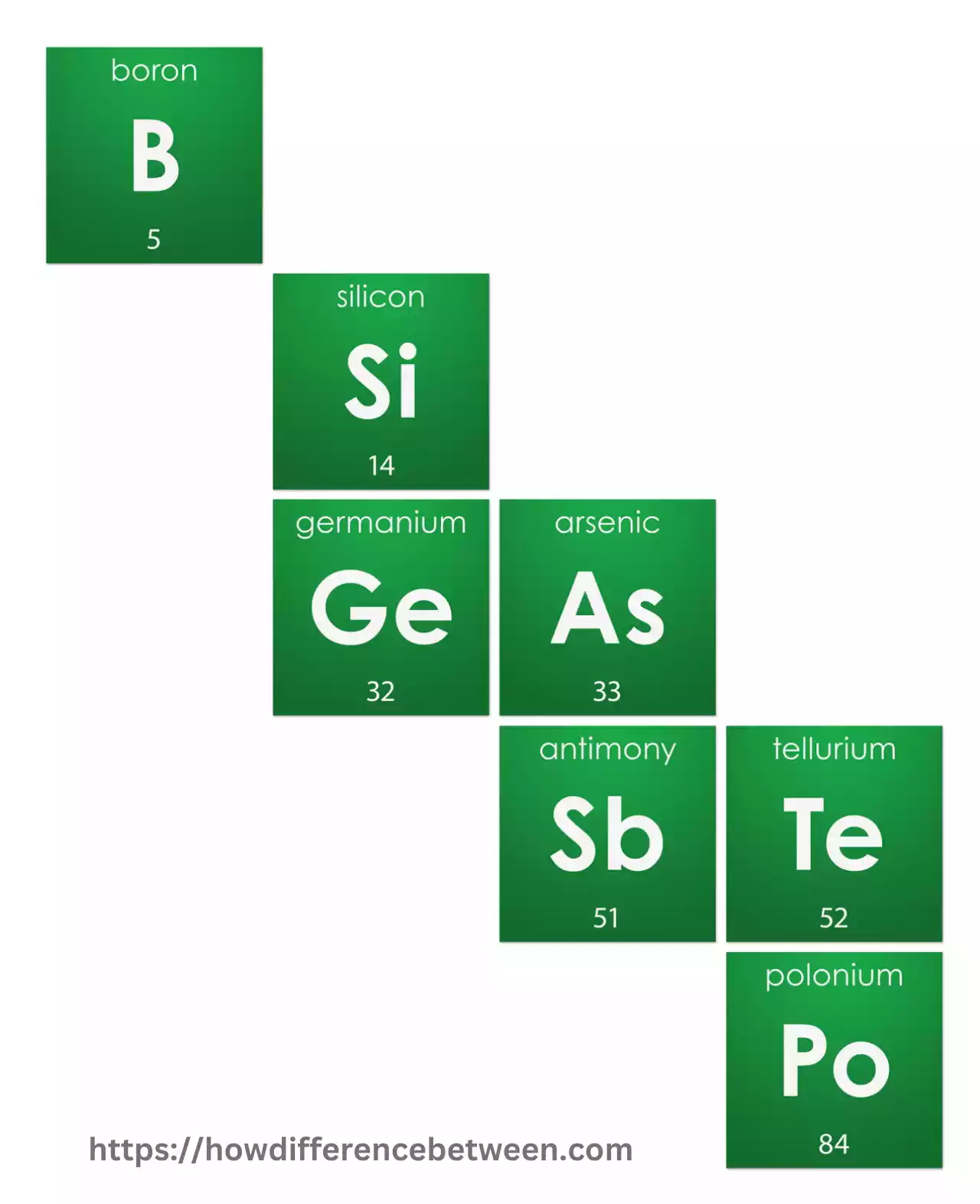
Tellurium, antimony, boron, and arsenic are metalloid elements used practically in several applications such as glass production and semiconductor material development.
Metalloids play an essential role in technological progress, particularly within electronics, telecom and materials science fields.
PropertiesMetalloidsloidssemi-metals or semi-metals as they are also called, have a mixture that parties laterality both metal and non-metal characteristics.
Here are some of the key properties of metalloids:
- Intermediate Conductivity: The conductivity of metalloids is intermediate between metals and non-metals. Although they are not as good conductors of electricity as metals, they can still conduct electricity to some degree. They are useful for semiconductor applications because of this property.
- Variable Luster: Metalloids have a variety of lusters, depending on their form and the element they contain. Metalloids such as arsenic can have a metallic look, whereas others, like silicone, will have a non-metallic one.
- Brittle and Lack of Malleability: Metalloids, unlike metals, are generally malleable and brittle. They cannot be deformed easily without breaking. Metalloids tend to fracture more than plastically deform like metals. This property limits the use of these materials in certain structural applications.
- Semiconductivity: Semiconductivity is a property that Metalloids possess. Under certain conditions, they can conduct electricity but not as well as metals. They are used to make electronic components like transistors, diodes, and integrated circuits because of this property.
- Variable Melting and Boiling Points: The melting and boiling point of metalloids can vary depending on their specific elements. Metalloids such as germanium and silicon have high melting points while others like boron have lower melting temperatures.
- Amphoteric Behavior: Metalloids exhibit both acidic as well as basic behavior. They can react both with bases and acids to produce complex ions. This is a behavior intermediate between metals’ basic properties and non-metals acidic properties.
- Allotropy: Metalloids exist in various allotropes. This means they have different crystal forms or structures. Carbon, for example, is a metalloid that can exist in different forms, such as graphite or diamond. Each of these has different chemical and physical properties.
Metalloids are silicon (Si), germanium(Ge), antimony(Sb), tellurium(Te), and boron. Metalloids are used in many applications due to their unique properties, especially in electronics, semiconductors, and materials science.
Examples of Metalloids
Metalloids (or semi-metals as they’re commonly known) are elements that fall between metals and nonmetals in terms of properties.
Here are a few metalloids examples:
- Silicon (Si): Silicon is one of the best-known metalloids, and an integral component of semiconductor technologies such as computer chips, solar panels, and other electronic devices.
- Germanium (Ge): Ge is used in semiconductor applications and shares many properties with silicon. Germanium can be found in infrared optical systems, fiber-optic cables, and transistors – among many other things.
- Arsenic: Arsenic is an arsenide metalloid with both metallic and nonmetallic properties. It’s used in manufacturing semiconductors, wood preservatives, and pesticides – although its highly toxic qualities necessitate extreme caution during handling and application.
- Antimony (Sb): Antimony can be found as an alloying agent in many materials including ceramics, batteries, and flame retardants.
- Tellurium (Te): Tellurium is an unusual metalloid with both metallic and nonmetallic properties that is used extensively for making solar panels, semiconductors, and various forms of glass products.
- Boron (B): Boron is an indispensable metalloid material with many applications in various fields and applications, from producing borosilicate glasses like Pyrex to producing semiconductor materials and various compounds.
- Selenium (Se): Selenium, an essential metalloid to human health in small doses, can also be found in photovoltaic panels, glass production processes, and electronic devices.
- Polonium: Polonium, an extremely radioactive metalloid used primarily in scientific research and thermoelectric power generation as well as some anti-static devices is an extremely valuable metalloid resource.
Metalloids have many varied uses and properties. From electronics manufacturing and semiconductor production to glass fabrication and automotive, metalloids can be seen everywhere from electronic components and semiconductors.
Comparison Chart
Here’s a comparison chart highlighting the key differences between metals and metalloids:
| Properties | Metals | Metalloids |
|---|---|---|
| Luster | Metallic luster (shiny, reflective) | Combination of metallic and non-metallic luster |
| Conductivity | Excellent conductors of heat and electricity | Intermediate conductivity, less efficient than metals |
| Malleability | Malleable and ductile | Generally brittle and lack malleability |
| Melting and Boiling Points | High melting and boiling points | Variable melting and boiling points |
| Reactivity | Varying reactivity | Variable reactivity |
| Oxidation States | Tend to form positive ions (cations) | Variable oxidation states |
| Allotropy | Generally do not exhibit allotropy | Some metalloids can exhibit allotropy |
| Placement on Periodic Table | Left side and middle | Along the “staircase” separating metals and non-metals |
| Applications | Construction, electrical wiring, transportation, various industries | Semiconductor industry, glass production, electronics |
Please note that this is a general comparison, and there may be variations and exceptions within each category of metals and metalloids.
Applications of Metals and Metalloids
Metals and metalloids have applications across numerous industries. Here are just a few common applications of each.
Metal Applications:
- Construction: Building with steel, aluminum, and copper has long been used in construction as structural elements, roofing materials, facade materials, and reinforcement owing to their long lifecycle and corrosion resistance.
- Transportation: Metals play an integral part in the transportation industry. From lightweight properties like aluminum alloy and steel alloy to structural integrity properties used by auto manufacturers to construct vehicles such as autos, ships, and trains – metals play an essential part.
- Electrical and Electronics: Metals with high conductivity such as copper and aluminum make for ideal electrical wiring and transmission cables, while their electrical conductivity makes silver and gold suitable components in electronic circuitry and components.
- Manufacturing: Metals can be utilized in numerous manufacturing processes such as metalworking and machining. Metal products range from appliances, consumer goods, and machinery,d tools; all crafted using this versatile medium.
- Packaging: Aluminum and steel containers are popular due to their strength, corrosion resistance, and recyclable nature – qualities that aluminum cannot match.
Metals have long been used as energy production equipment. Steel is commonly employed for wind turbines and offshore oil rigs while copper and silver alloys such as solar panel material due to their conductivity properties.
Applications for Metalloids
- Semiconductor Industry: Silicon and germanium metalloids play an essential role in semiconductor industries due to their semiconducting qualities; due to this feature they serve as bases for computer chips, integrated Circuits, and electronic products.
- Glass Production: Metaloids such as boron or silicon are employed in glass manufacturing processes, with compounds made up of these elements used to enhance its thermal and chemical resistance and add durability. Silicon dioxide (silica), another common glass component, may also be added.
- Electronics and optics: Metaloids such as arsenic and antimony can be found in electronics components, optical devices, and infrared sensors used in producing electronic devices and sensors. Their semiconducting qualities help in the creation of transistors, sensors, and diodes used extensively across electronics manufacturing processes.
- Catalysts: Metaloids such as antimony, bismuth, and arsenic can serve as catalysts to accelerate or regulate chemical reactions across industries like petrochemicals and pharmaceuticals.
Silicon and boron are commonly used as alloying agents in steel production to give certain steels and metals desirable properties, like heat resistance or hardness enhancements. - Biomedical Applications: Metaloids like selenium or germanium have applications in biomedicine. Selenium has long been employed for imaging techniques as well as nutritional supplement use; germanium might even find use in treating cancer or for drug delivery purposes.
Here are just a few applications of metals and metalloids, from their use in many industries to contributing to technological progress that we rely on daily.
Future Perspectives
Metals and metalloids offer great potential due to technological developments, innovation, and sustainability initiatives.
Below are a few key areas for consideration.
- Advanced Materials Development: Researchers dedicate themselves to creating novel alloys and materials with improved properties for use across industries like aerospace, automotive, or energy production. Their goal is to make materials stronger, lighter, and more durable as well as offer increased functionalities designed to meet industry requirements such as aerospace.
- Sustainable and eco-friendly solutions: Current trends stress minimizing environmental impact through responsible metal extraction, processing, and waste disposal practices. Circular economy approaches are becoming more popular to decrease resource depletion while simultaneously encouraging optimal utilization.
- Metals and Metalloids: Essential Components in Energy Storage and Conversion Metals and metalloids play an essential part in developing advanced energy storage technology, including high-capacity battery packs for electric cars and grid-scale storage systems. Silicon, in particular, continues to play an essential part in improving photovoltaic cell efficiencies for solar energy conversion.
- Nanotechnology and Miniaturization: Metals and metalloids play an integral part in nanotechnology, used for producing nanoparticles and nanowires that advance electronics, sensing technologies, catalysis processes, and biomedical technologies.
- Semiconductor Industry Advancements: The semiconductor industry relies heavily on metalloids like silicon for growth. As technology progresses, consumers’ need for smaller, quicker, and more energy-efficient electronics increases – leading to improvements in semiconductor production as well as research of novel materials.
- Biomedical Applications: Metaloids such as selenium and germanium have great promise as medicine and healthcare technologies, with studies being done into their therapeutic potential as drug delivery systems, imaging agents, or targeted therapies.
- Sustainable Water and environmental solutions: Metaloids such as arsenic have played an instrumental role in developing technologies for water treatment and environment remediation, with particular attention paid to finding cost-effective methods of eliminating contaminants for safe drinking water supplies.
- Quantum Technologies: Metaloids such as silicon are being studied to see how they might be utilized for quantum computing applications, which promise to revolutionize computing by solving complex issues from various fields simultaneously.
Metals and metalloids will continue to play an integral part in many fields, from materials science and sustainable practices to energy technologies and quantum technology, and nanotechnology. Their advancement will help address global challenges while improving our quality of living.
Conclusion
Metals and their alloys play an essential part in our everyday lives, from construction and electronics to transportation and more. Metals stand out for their superior conductivity, malleability, and strength as they find widespread application across industries including construction, electronics, and transportation – not forgetting industries such as semiconductor production, glass making, or catalysis – in which metalloids also find applications.
Metals and metalloids play an essential role in infrastructure development, energy production, and technological progress. Their future promises look bright; research and innovation focused on advanced materials with sustainable practices such as energy storage and nanotechnology are promising areas.