Cubic Close and Hexagonal Close Packing structures play a pivotal role in various industries and disciplines.
Brief explanation of close packing in solids
Close packing in solids refers to the practice of densely packed atoms, ions, or molecules into an arrangement with minimal empty spaces between them; it’s an integral concept in materials science and solid-state physics that maximizes packing efficiency – that is the ratio of the volume occupied to the total volume occupied compared with its total volume occupied. It aims to maximize packing efficiency through density.
Close packing involves aligning atoms in an organized pattern to form layers or planes in which each one occupies one space on top of another in an effort to minimize empty spaces between atoms, thus producing a closer-packed structure with minimal empty spaces between atoms – this arrangement determines important properties such as mechanical strength, electrical conductivity and optical properties of materials.
Close-packing structures come in two broad categories, hexagonal close packing (HCP) and cubic close packing (CCP). Both structures involve placing the atoms into repetitive patterns with differences depending on stacking sequence and cell symmetry.
Understanding close packing in solids is fundamental for understanding crystal structures, alloy formation, and material behavior under different environmental conditions. It offers insight into the arrangement and interactions among atoms which contributes to improved understanding of material properties as well as potential applications.
Importance of Cubic Close and Hexagonal Close Packing
Close packing plays an essential part in understanding crystal structures – which involve ordering arrangements of atoms, ions, and molecules found within solids – through close packing of molecules or atoms into ordered arrangements in solids.
Here are several reasons for its significance when studying crystal structures:
- Determination of Unit Cell: Close packing provides the foundation for defining a unit cell in crystal lattices; this repeating structural unit represents the smallest repeating arrangement of atoms which generates crystal structures and thus can be identified and described concisely and systematically by understanding close packing arrangements.
- Predicting Crystal Symmetry: Close packing arrangements exhibit specific symmetries that play an essential part in characterizing crystal structures. These symmetries often reflect in crystallographic axes, planes and operations of symmetry operations present within crystal lattices – thus helping determine its type (such as cubic, hexagonal or tetragonal ) as well as point group symmetry of its crystal system.
By analyzing close packing patterns closely enough, one can predict elements and operations present within crystal lattices which allow one to predict symmetry elements present within crystal lattices; hence helping determine its crystal system type as well as point group symmetry of its point group symmetry of point group symmetry of its point group symmetry of its point group symmetry of its point group symmetry system and point group symmetry point group symmetry point group symmetry point group symmetry of crystal system. - Understanding Crystallographic Directions and Planes: Close packing arrangements provide an effective framework for defining crystallographic directions and planes. Crystallographic directions refer to lines within a crystal lattice while crystallographic planes refer to specific arrangements of atoms within crystal structures. By studying close packing patterns closely enough, recognizing and understanding these directions becomes simpler – knowledge essential for crystallography, mineralogy and materials science research.
- Predicting Packing Efficiency: Close packing provides insight into the packing efficiency of crystal structures. Packing efficiency measures how efficiently atoms occupy their volume relative to total unit cell volume occupied, so different close packing arrangements have different packing efficiencies that help understand density and stability as well as potential sites of interstitial atoms or defects in crystals.
- Influence on Material Properties: Close packing arrangements have an undeniable influence on the physical, mechanical, and optical properties of materials. Their dense packing arrangement influences density, hardness, strength of crystal structure as well as optical properties like refractive index and transparency – an understanding of which is vital in order to predict or manipulate their properties according to crystal structures.
Close packing serves as a crucial foundational concept in understanding crystal structures. It helps identify unit cells, predict crystal symmetry and direction planes, analyze packing efficiency as well as correlate close packing arrangements with material properties; ultimately becoming the basis of solid-state physics, materials science crystallography mineralogy studies.
What is Hexagonal Close Packing (HCP)?
Hexagonal close packing (HCP) is a type of crystal lattice structure commonly found in metals and some non-metallic elements. It is a closely packed arrangement of atoms, ions, or spheres in a crystalline lattice. In HCP, the atoms are arranged in a repeating pattern that maximizes the efficient use of space.
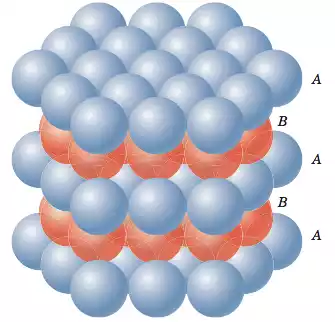
Key features of hexagonal close packing (HCP) include:
- Unit Cell: The basic repeating unit of the HCP lattice is a hexagonal prism with a top and bottom hexagonal face, and two additional parallel hexagonal faces connecting the top and bottom faces. The unit cell contains three layers of atoms.
- Atom Arrangement: Atoms are arranged in closely packed layers. The second layer is directly above the first layer, and the third layer is placed above the first layer but is aligned with the valleys of the second layer. This stacking sequence is denoted as ABABAB… where A and B represent different layers.
- Coordination Number: Each atom in an HCP lattice is in contact with 12 nearest neighboring atoms. This results in a coordination number of 12.
- Packing Efficiency: HCP has a packing efficiency of about 74%. This means that approximately 74% of the available space within the lattice is occupied by atoms.
- Closest Packing: HCP is a type of closest packing, which means that the atoms are positioned as close to each other as possible without overlapping.
- Crystallography: The HCP structure has a high degree of symmetry. It belongs to the hexagonal crystal system and has a space group denoted as P6₃/mmc.
- Examples: Some common materials that adopt the HCP structure include metals like magnesium (Mg), zinc (Zn), cadmium (Cd), and titanium (Ti), as well as certain non-metallic elements and compounds.
HCP structures have specific properties and behaviors that arise from their atomic arrangement. They are important in materials science and engineering because they influence the mechanical, thermal, and electrical properties of materials. Understanding crystal lattice structures like HCP is crucial for designing and engineering materials with desired characteristics for various applications.
What is Cubic Close Packing (CCP)?
Cubic close packing (CCP), also known as face-centered cubic (FCC) packing, is a common crystal lattice structure found in metals and some other materials. It is a closely packed arrangement of atoms, ions, or spheres in a repeating pattern within a three-dimensional lattice.
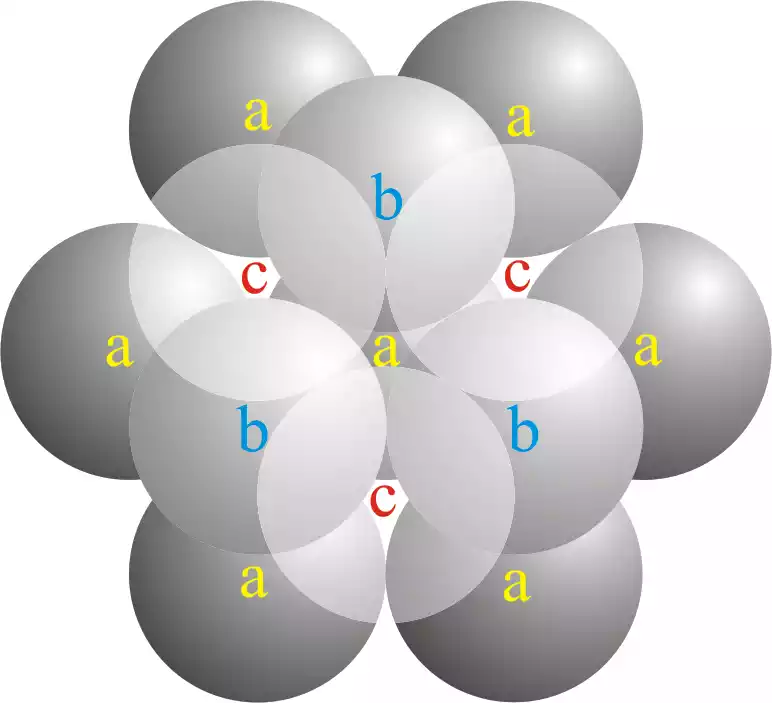
Key features of cubic close packing (CCP) include:
- Unit Cell: The basic repeating unit of the CCP lattice is a cube. The atoms are positioned at the corners of the cube and at the center of each face of the cube.
- Atom Arrangement: In CCP, the atoms are arranged in layers. The second layer is directly above the first layer, and the third layer is placed above the first layer but is shifted to align with the positions of atoms in the first layer. This stacking sequence is denoted as ABCABC… where A, B, and C represent different layers.
- Coordination Number: Each atom in a CCP lattice is in contact with 12 nearest neighboring atoms. This results in a coordination number of 12.
- Packing Efficiency: CCP has a packing efficiency of about 74%, meaning that approximately 74% of the available space within the lattice is occupied by atoms.
- Closest Packing: CCP is a type of closest packing, where the atoms are positioned as close to each other as possible without overlapping.
- Crystallography: The CCP structure belongs to the cubic crystal system and has a space group denoted as Fm-3m.
- Examples: Common materials that adopt the CCP structure include metals like copper (Cu), silver (Ag), gold (Au), and aluminum (Al).
Cubic close packing is an important concept in materials science and solid-state physics. The arrangement of atoms in CCP structures contributes to the mechanical, thermal, and electrical properties of materials. Understanding these structures is essential for designing and engineering materials with specific properties for various applications.
Differences Between Cubic Close and Hexagonal Close Packing
Cubic close packing (CCP) and hexagonal close packing (HCP) are two common arrangements of atoms or spheres in crystalline structures.
While they both involve closely packed arrangements, they have distinct differences:
1. Unit Cell Shape:
- CCP: In cubic close packing, the unit cell is cubic, with atoms/spheres placed at the corners and in the center of each face.
- HCP: In hexagonal close packing, the unit cell is hexagonal, with atoms/spheres placed at the corners and in the center of the top and bottom faces.
2. Atom/Sphere Arrangement:
- CCP: In CCP, the layers of atoms/spheres stack in an ABCABC… pattern, where each layer is directly above the one below it.
- HCP: In HCP, the layers of atoms/spheres stack in an ABAB… pattern, where each layer is offset and aligned with the “valleys” of the layer below it.
3. Coordination Number:
- CCP: The coordination number in CCP is 12, meaning that each atom is in contact with 12 neighboring atoms.
- HCP: The coordination number in HCP is also 12, with each atom in contact with 12 neighboring atoms.
4. Packing Efficiency:
- CCP: CCP has a packing efficiency of 74%, meaning that about 74% of the available space is occupied by atoms.
- HCP: HCP also has a packing efficiency of 74%.
5. Stacking Sequence:
- CCP: In CCP, the stacking sequence of the layers is cubic, and the atoms are arranged in a face-centered manner.
- HCP: In HCP, the stacking sequence is hexagonal, and the atoms are arranged in a hexagonal pattern.
6. Crystallography:
- CCP: CCP is more common in metallic structures, particularly in fcc (face-centered cubic) lattices.
- HCP: HCP is common in some metals, as well as in non-metallic elements like some close-packed minerals.
7. Packing Direction:
- CCP: In CCP, the packing direction is the same in all three dimensions.
- HCP: In HCP, the packing direction is different between the vertical and horizontal planes.
8. Example Materials:
- CCP: Examples of materials with CCP structure include metals like copper, silver, and gold.
- HCP: Examples of materials with HCP structure include metals like magnesium, zinc, and titanium.
Both cubic close packing (CCP) and hexagonal close packing (HCP) are closely packed arrangements of atoms or spheres, but they differ in terms of unit cell shape, stacking sequence, and packing direction. These differences contribute to the distinct characteristics and properties of materials that adopt these structures.
Similarities Between Hexagonal Close Packing and Cubic Close Packing
Hexagonal close packing (HCP) and cubic close packing (CCP), also known as face-centered cubic (FCC) packing, are two closely related crystal lattice structures that share several similarities:
- Packing Efficiency: Both HCP and CCP have the same packing efficiency of about 74%, meaning that approximately 74% of the available space within the lattice is occupied by atoms. This high packing efficiency results in relatively dense and compact arrangements of atoms.
- Coordination Number: Both HCP and CCP structures have the same coordination number of 12. Each atom in the lattice is in contact with 12 nearest neighboring atoms. This coordination number contributes to the strong bonding and stability of the lattice.
- Closest Packing: Both HCP and CCP are examples of closest packing, where atoms are positioned as closely as possible to each other without overlapping. In both structures, every atom is surrounded by the maximum possible number of nearest neighbors, contributing to the efficient use of space.
- Stacking Sequence: Both HCP and CCP structures involve stacking layers of atoms. In both cases, the stacking sequence is based on a repeating pattern of layers. While the specific stacking sequence differs between the two structures, the underlying concept of arranging layers is similar.
- High Symmetry: Both HCP and CCP structures exhibit a high degree of symmetry. HCP belongs to the hexagonal crystal system, while CCP belongs to the cubic crystal system. The symmetrical arrangement of atoms contributes to the unique properties of these structures.
- Materials: Both HCP and CCP are commonly found in metallic materials. Many metals can adopt either HCP or CCP structures depending on the specific conditions, such as temperature and pressure.
Despite these similarities, it’s important to note that HCP and CCP structures have distinct differences in terms of their unit cell shape, atom arrangement, crystallography, and stacking sequences. These differences give rise to unique properties and behaviors that differentiate the two structures.
Applications and significance
Applications and Significance of HCP and CCP Structures:
Industrial Applications for HCP Metals:
- HCP: Magnesium and titanium HCP structures have become popular choices among aerospace, automotive and medical implant industries for their high strength-to-weight ratios, making these metals widely employed across several fields of endeavor. Their applications also extend into electronic devices, batteries and medical implants among many others.
- CCP: metals such as copper and silver are widely utilized for electrical wiring, conductors and electronic components due to their excellent electrical and thermal conductivity properties. Their versatility in various industries makes these structures worth using in many different sectors.
Formation:
- HCP: Structures play an integral part in alloy formation. By adding small amounts of other elements to HCP metals, addition can change their properties such as increasing strength or corrosion resistance – properties which often find applications within aircraft components and engine parts.
- CCP: structures play a crucial role in alloy formation. Many popular alloys such as brass (copper-zinc) and bronze (copper-tin), feature CCP structures for strength, corrosion resistance and machinability properties.
Material Properties of HCP Structures:
- HCP: structures exhibit anisotropic properties, meaning their characteristics depend on which direction you look in. This anisotropy can be traced back to their layers of atoms in HCP metals; understanding this structure allows us to predict and manipulate mechanical, thermal and electrical properties effectively.
- CCP: structures feature isotropic properties that remain uniform in all directions – an asset when consistent properties are desired for applications like electrical conductors and heat exchangers.
Crystallography and Materials Science offer further insights:
- HCP and CCP structures play an essential role in crystallography, acting as model structures to examine crystal symmetry, crystallographic directions and planes, material properties under different environmental conditions and behavior of materials under various circumstances. Being knowledgeable of HCP and CCP structures gives materials scientists a basis to characterize and analyze various crystal structures encountered.
Fundamental Research and Exploration:
- Research of HCP and CCP structures remains an active area of investigation, as scientists investigate new materials and alloys with these structures, investigating their properties, behavior and possible applications. Knowledge gained through such investigations enhances our knowledge of close packing phenomena, crystal structures, relationships between structure and properties as well as new applications of such research findings.
HCP and CCP structures play a pivotal role in various industries and disciplines. Their significance includes alloy design, understanding material properties, crystallography advancements and fundamental research advances; all while playing an instrumental role in shaping materials’ properties and behavior allowing the creation of innovative materials suitable for various uses.
Ending
Cubic Close and Hexagonal Close Packing are fundamental arrangements that dictate the properties of various materials. Their distinct stacking sequences lead to unique physical and chemical attributes, enabling a wide range of applications across industries. As we continue to explore the intricate world of crystal structures, we unveil opportunities to revolutionize technology and enhance our understanding of the materials that shape our world.