Convalescent Plasma and Monoclonal Antibodies represent two distinct, yet critically important, therapeutic tools in the realm of infectious disease management and beyond. Convalescent plasma, sourced from individuals who have recovered from an infection, offers a rich mixture of antibodies that have the potential to aid others in their fight against the same disease. On the other hand, monoclonal antibodies are lab-engineered marvels, designed to target specific components of a pathogen with precision.
While both have their roots in the immune system’s natural defenses, their origins, mechanisms of action, and applications can differ considerably, offering unique advantages and challenges in medical treatments.
Definition of Convalescent Plasma
Convalescent plasma refers to the liquid component of blood that is collected from patients who have recovered from an infectious disease. This plasma is rich in antibodies that have been produced by the immune system to fight off the disease. When transfused into patients still battling the disease, these antibodies can aid in their recovery by providing passive immunity. This means the recipient benefits from the donor’s immune response without having to generate their own immune response.
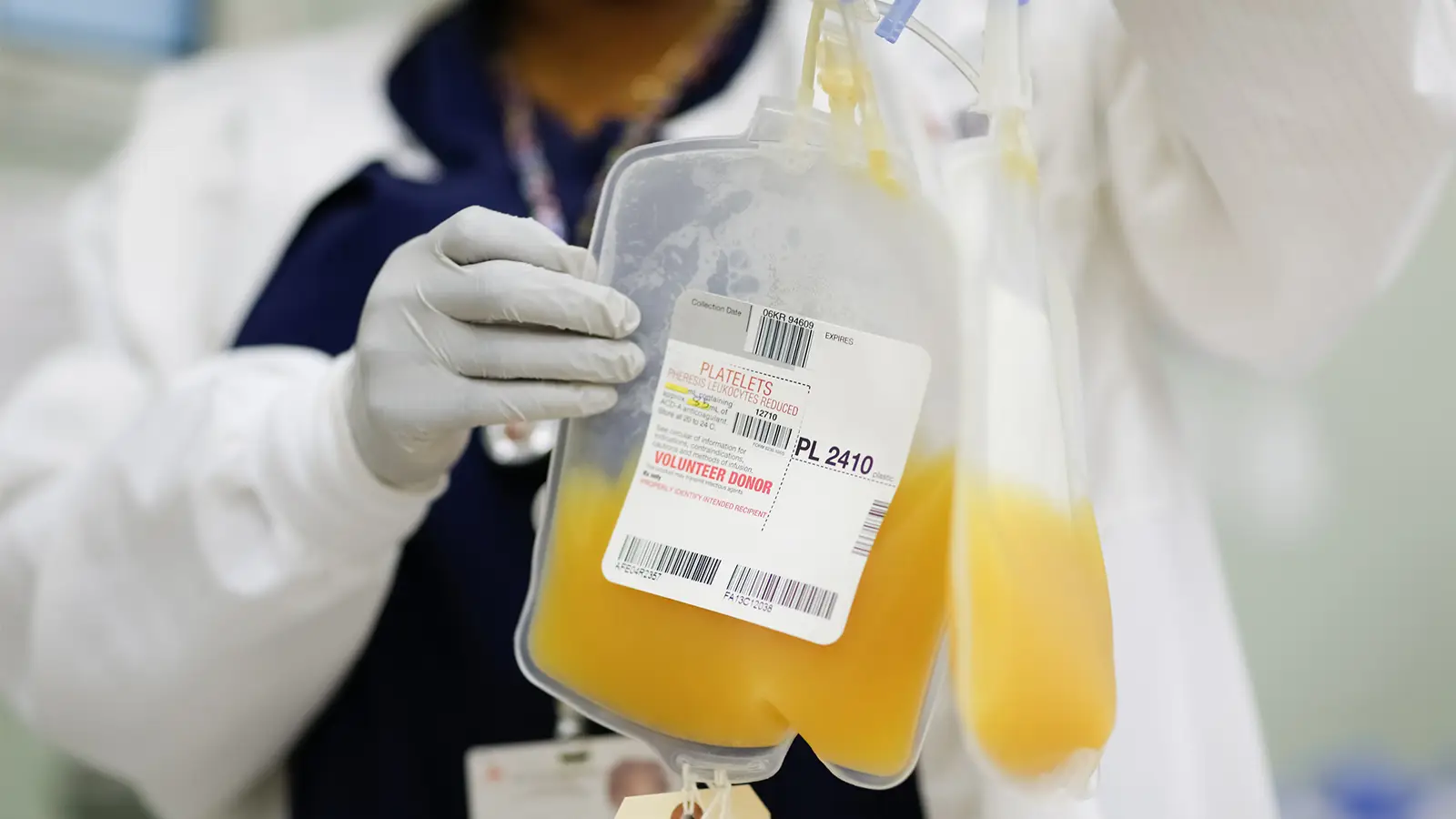
Historically, convalescent plasma has been used as a therapeutic approach in various outbreaks, including the Spanish Flu, SARS, MERS, and more recently, COVID-19. The premise behind its use is to harness the disease-fighting capabilities (antibodies) from recovered individuals to help those who are still affected by the disease.
Definition of Monoclonal Antibodies
Monoclonal antibodies (mAbs) are laboratory-produced molecules designed to mimic the immune system’s ability to fight off harmful pathogens, such as viruses and bacteria. These antibodies are made by creating identical immune cells, all clones of a unique parent cell, hence the term “monoclonal.” They can be specifically engineered to bind to a singular target, such as a specific protein on the surface of a pathogen or a cancer cell.
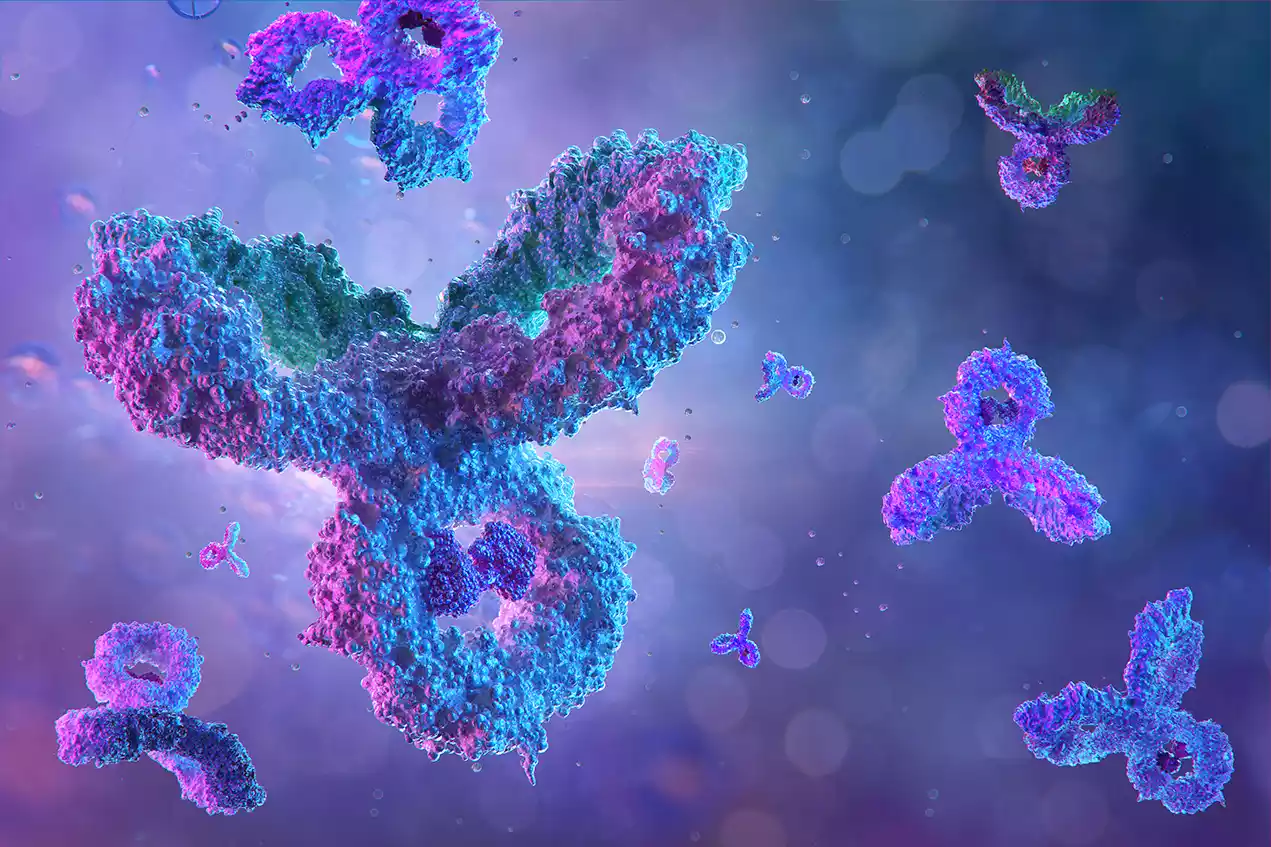
Monoclonal antibodies have a wide range of therapeutic applications, including the treatment of cancers, autoimmune disorders, and infectious diseases. They function by either neutralizing the target organism or molecule directly or by marking it for destruction by other cells of the immune system.
Due to their specificity and ability to be engineered for a particular purpose, monoclonal antibodies have become invaluable tools in both medicine and research.
Comparison Table of Convalescent Plasma and Monoclonal Antibodies
Here’s a comparison table between Convalescent Plasma and Monoclonal Antibodies:
| Feature/Criteria | Convalescent Plasma | Monoclonal Antibodies |
|---|---|---|
| Source | Blood of recovered patients | Laboratory-produced |
| Composition | Mixed antibodies from donor’s immune response | Single, specific, engineered antibody |
| Production | Dependent on donors | Scalable and controlled manufacturing process |
| Standardization | Varies (based on donor’s immune response) | Highly standardized and consistent |
| Mechanism of Action | Provides passive immunity with diverse antibodies | Targets specific proteins or cells directly |
| Usage History | Used in various outbreaks historically | Broad therapeutic applications, including cancer and auto-immune diseases |
| Availability | Limited (dependent on recovered donors) | More consistent (depends on manufacturing capabilities) |
| Potency and Specificity | Variable potency and specificity | High potency and specificity |
| Administration | Blood transfusion | Typically infusion or injection |
| Cost | Relatively low (primarily collection costs) | Often high due to R&D, manufacturing complexities |
| Potential Side Effects | Transfusion-related complications | Infusion reactions, potential off-target effects |
This table provides an overview of the primary differences between convalescent plasma and monoclonal antibodies, but it’s important to note that both therapies have their advantages and limitations. The best choice often depends on the specific context and clinical scenario.
Benefits of Convalescent Plasma and Monoclonal Antibodies
Both Convalescent Plasma and Monoclonal Antibodies offer unique therapeutic benefits in the realm of medicine. Here’s a comparison of the benefits each provides:
Convalescent Plasma
- Immediate Immunity: Once transfused, convalescent plasma can offer immediate passive immunity to the recipient, especially useful in acute or severe cases where there’s no time for the patient’s immune system to develop its own response.
- Broad Spectrum: Unlike monoclonal antibodies which are specific to a single target, convalescent plasma contains a mixture of antibodies. This means it might target multiple parts of the pathogen or disease-causing agent, potentially preventing escape mutants or resistant strains.
- Natural Source: Derived from human donors, the plasma is a natural product. This can sometimes lead to fewer side effects as compared to synthetic drugs.
- Versatility: Historically, it has been used as an emergency intervention during outbreaks of novel diseases when other treatments aren’t available. Its broad-spectrum nature makes it adaptable to many pathogens.
- Established Procedures: Blood plasma transfusion is a well-established medical procedure, and healthcare systems globally have infrastructure in place for this.
Monoclonal Antibodies
- High Specificity: Monoclonal antibodies are designed to target a specific molecule. This specificity ensures that the therapeutic action is directed precisely where it’s needed, minimizing potential off-target effects.
- Consistency and Purity: Being produced in controlled laboratory environments, monoclonal antibodies are consistent in their composition and action, as opposed to convalescent plasma which can vary between donors.
- Customizability: Scientists can design and modify monoclonal antibodies for specific needs, optimizing them for better efficacy, reducing potential side effects, or altering them to last longer in the body.
- Widespread Therapeutic Applications: As discussed earlier, monoclonal antibodies have found applications in a range of diseases, from cancers to infectious diseases to autoimmune disorders.
- Fewer Transfusion-related Risks: Monoclonal antibodies don’t carry the risks associated with blood product transfusions, such as allergic reactions or transmission of blood-borne pathogens.
- Scalability: Once a monoclonal antibody is developed, it can be produced in large quantities in a consistent manner, which is beneficial for widespread distribution and use.
While both treatments have their own set of advantages, the choice between them depends on the specific clinical scenario, the disease being targeted, and the available evidence supporting their efficacy and safety.
Limitations and Concerns
Like all therapeutic interventions, both convalescent plasma and monoclonal antibodies come with limitations and potential concerns:
Convalescent Plasma
- Variability: The concentration and efficacy of antibodies can vary greatly between donors, depending on factors like the severity of their illness, their immune response, and the time since recovery.
- Supply Limitations: The availability of convalescent plasma depends on the number of recovered patients willing to donate and can be especially challenging during the early stages of a disease outbreak.
- Safety Concerns: While blood donation and transfusion processes are rigorous, there’s still a risk, albeit very low, of transmitting infections. Additionally, there’s the possibility of transfusion reactions, which can range from mild allergic reactions to severe reactions like transfusion-associated lung injury (TRALI).
- Efficacy Questions: The efficacy of convalescent plasma is not always consistent and can be variable for different diseases or conditions.
- Short-lived Immunity: The immunity provided by convalescent plasma is passive and wanes over time as the antibodies degrade.
Monoclonal Antibodies
- Costly Production: The production of monoclonal antibodies is often expensive, which can translate to high costs for patients and healthcare systems.
- Resistance Development: Pathogens, especially viruses, can mutate over time. A mutation in the target of a monoclonal antibody can render it less effective or ineffective.
- Side Effects: While they are designed to be specific, mAbs can still have side effects, some of which can be severe, depending on the target and the mechanism of action.
- Administration: Many monoclonal antibodies need to be administered intravenously, which requires clinical settings and can be less convenient than oral medications.
- Potential Immunogenicity: Despite being designed for human use, some monoclonal antibodies can still provoke an immune response, potentially reducing their efficacy or leading to allergic reactions.
- Specificity Limitation: While specificity is an advantage, it’s also a limitation. If a disease has multiple causative agents or pathways, a monoclonal antibody might not be as effective as broader-acting treatments.
- Storage and Stability: Some monoclonal antibodies require specific storage conditions, which can complicate their distribution and use, especially in low-resource settings.
Both convalescent plasma and monoclonal antibodies have shown to be valuable tools in the medical arsenal, but they aren’t without challenges. It’s crucial for clinicians and patients to weigh the benefits against the limitations and concerns when considering these treatments.
Real-world Applications and Case Studies
Convalescent plasma and monoclonal antibodies have both been used in various therapeutic contexts. Here are some real-world applications and case studies for each:
Convalescent Plasma:
- Spanish Flu (1918 Influenza Pandemic):
- Application: In the absence of vaccines or antiviral drugs, convalescent plasma from recovered patients was used as a treatment option during the 1918 influenza pandemic. Some studies from that era suggested a reduction in mortality in those treated with convalescent plasma.
- Ebola Virus Outbreak (2013-2016):
- Case Study: Convalescent plasma was used experimentally as a treatment for patients during the Ebola outbreak in West Africa. The results were mixed, with some studies suggesting a potential benefit and others indicating no significant effect.
- COVID-19 Pandemic:
- Application: With the emergence of SARS-CoV-2, several countries authorized the emergency use of convalescent plasma from recovered COVID-19 patients, especially in the early stages of the pandemic. Some studies indicated potential benefits, especially when administered early and with high neutralizing antibody titers.
Monoclonal Antibodies:
- Cancer Therapy:
- Application: Rituximab, a monoclonal antibody, has been widely used to treat certain types of non-Hodgkin lymphomas and chronic lymphocytic leukemia by targeting CD20 on B cells.
- Autoimmune Diseases:
- Application: Monoclonal antibodies like adalimumab and infliximab have been used to treat diseases such as rheumatoid arthritis, Crohn’s disease, and ulcerative colitis by targeting specific inflammatory factors.
- Respiratory Syncytial Virus (RSV) in Infants:
- Case Study: Palivizumab is a monoclonal antibody used as a prophylactic measure against severe RSV infections in high-risk infants.
- COVID-19 Pandemic:
- Application: Monoclonal antibodies like bamlanivimab and etesevimab (Eli Lilly) and casirivimab and imdevimab (Regeneron) received emergency use authorizations in various countries for the treatment of mild to moderate COVID-19 in certain patients.
- Case Study: Former U.S. President Donald Trump was treated with Regeneron’s monoclonal antibody cocktail when he contracted COVID-19, bringing significant attention to this form of treatment.
Both convalescent plasma and monoclonal antibodies represent the broader theme of using the body’s immune response as a therapeutic tool, either by harvesting it from recovered individuals or by creating it synthetically in labs. Each application or case study provides valuable insights into the potential, challenges, and complexities of these treatments across different diseases and contexts.
The Future of Convalescent Plasma and Monoclonal Antibodies
The use of convalescent plasma and monoclonal antibodies has drawn considerable attention, especially during the COVID-19 pandemic. As we think about their future, several themes and predictions emerge:
Convalescent Plasma:
- Repository Creation: There may be efforts to create plasma banks from survivors of various diseases, ensuring a ready supply for potential future outbreaks.
- Standardization and Potency: As the science progresses, there might be better guidelines to ascertain the potency of convalescent plasma, ensuring that only high-titer or antibody-rich plasma is used.
- Combinational Therapies: Convalescent plasma might be used in combination with other therapies to increase the efficacy of treatment, especially in severe cases.
- Research on Mechanisms: Continued research on how convalescent plasma impacts recipients at the cellular and molecular level can pave the way for more targeted therapies.
Monoclonal Antibodies:
- Customization against Variants: As viruses mutate, there will be a need to continually adapt monoclonal antibodies. The ability to rapidly design, test, and produce monoclonal antibodies against emerging variants will be crucial.
- Prophylactic Use: Beyond treatment, monoclonal antibodies might be used as temporary prophylactics, especially for high-risk populations who can’t receive vaccines or before vaccines become available.
- Technological Advancements: Advancements in biotechnology could reduce the cost and time required to produce monoclonal antibodies.
- Delivery Innovations: Research may lead to more convenient delivery methods, eliminating or reducing the need for intravenous infusions.
- Broader Applications: Monoclonal antibodies have therapeutic potential beyond infectious diseases, including cancer, autoimmune disorders, and other conditions. Their future might see expanded roles in various therapeutic domains.
- Combination Therapies: Using a cocktail of several monoclonal antibodies (like the combination used to treat former U.S. President Donald Trump during his bout with COVID-19) can be more effective than a single monoclonal antibody, reducing the chances of the virus developing resistance.
Overarching Themes:
- Regulatory Scrutiny: As with all medical interventions, both convalescent plasma and monoclonal antibodies will continue to be under rigorous scrutiny for efficacy, safety, and ethical considerations.
- Global Accessibility: Ensuring that treatments are accessible to all, especially low and middle-income countries, will be a significant challenge. International cooperation and efforts by global health organizations will be essential.
- Public Perception and Acceptance: The public’s trust in medical interventions is vital. Continuous efforts will be needed to educate the public and counter misinformation.
While the future holds promise for both convalescent plasma and monoclonal antibodies, their success will depend on a combination of scientific advancements, regulatory decisions, public perception, and the evolving landscape of infectious diseases and other health challenges.
Conclusion
Convalescent Plasma and Monoclonal Antibodies represent two distinct therapeutic strategies, each with its unique advantages and challenges. Convalescent plasma, derived from recovered patients, offers a natural blend of antibodies, providing immediate but temporary immunity. Its efficacy can be variable, and availability is donor-dependent. On the other hand, monoclonal antibodies are lab-engineered molecules targeting specific pathogens or proteins with high precision.
While they promise consistency and customizability, they come with concerns about cost, potential resistance, and side effects. Both tools, despite their limitations, have played significant roles in various medical scenarios and continue to be vital assets in the therapeutic landscape.