A brief explanation of the importance of glycerol and fatty acids in biological systems
Glycerol and fatty acids play vital roles in our biological systems and play an essential part of many processes,
so here is an outline of their significance:
- Energy Storage and Metabolism: Fatty acids provide our bodies with energy in abundance; beta-oxidation being one way by which they release large quantities of ATP – cells’ currency for energy release. Glycerol can then be converted to glycerol-3-phosphate which, through glycolysis, will generate further Energy Production.
- Structural Component: Fatty acids are key elements of lipids that compose their structure; specifically phospholipids, triglycerides cholesterol esters and phospholipids. Phospholipids provide crucial support to cells by helping create their membrane structure while simultaneously keeping membrane integrity intact and functioning effectively. These essential lipids also enable separation between cell layers within membranes by maintaining separation while still upholding integrity integrity of membranes.
- Insulation and Protection: Adipose tissue, composed largely of triglycerides, acts as an insulating layer against mechanical strain on organs as well as providing energy reserves when fasting or experiencing increased energy demands.
- Hormone Regulation: Certain fats play an integral part in both the production of hormones as well as their regulation. Arachidonic acid serves as an indispensable precursor for synthesizing prostaglandins which play key roles in biological processes including blood clotting, inflammation control and hormone balance regulation.
- Accumulation and Transport: Fat acids travel throughout the body in their bloodstream where they combine with transport proteins to form lipoproteins like chylomicrons or VLDL (Very Low-Density Lipoprotein), aiding with digestion of food while simultaneously transporting them directly into cells throughout our bodies.
- Essential Nutrients: Certain fats known as essential acids cannot be made by our bodies alone and require diet for production and supply; such as omega-3 and omega-6 fats which regulate brain and immune system functioning as well as inflammation control.
- Osmoregulation: Glycerol plays an essential role in keeping cell osmotic pressure balanced and helping limit excessive water loss or gain as the environment fluctuates. Being an inert solvent, glycerol helps limit excessive loss or gain of water within cells based on environmental changes and external conditions.
Understanding the role fatty acids and glycerol play in biological systems is paramount for understanding their effect on energy metabolism, structure integrity, hormone regulation and general bodily well-being.
What is Glycerol?
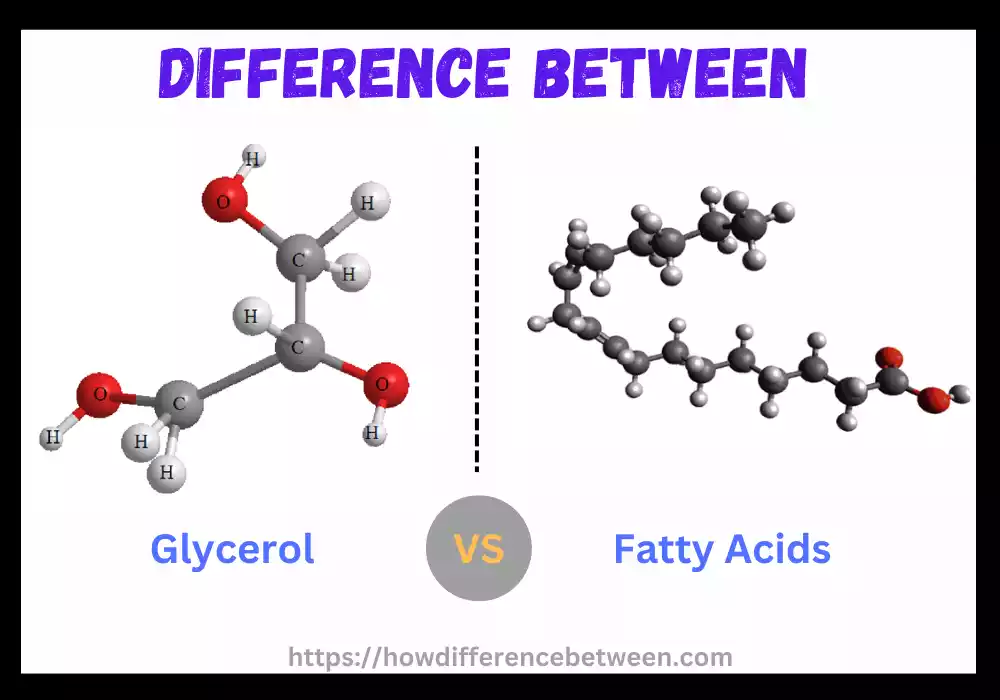
Glycerol (also referred to as Glycerin or glycerine) is an uncolored, odorless liquid belonging to organic compounds of the alcohol family that features as an inert trihydroxy sugar alcohol with three hydroxyl (OH) groups connected via carbon backbone – abundantly found both as part of various oils and fats as well as being produced through metabolic processes. It occurs naturally as well.
Glycerol has the molecular formula C3H8O3, with an approximate molecular weight of around 92.09 grams for each mole. Chemically speaking, its chemical structure includes three carbon chains each linked with a hydroxyl group and therefore bonding them together into triple carbon chains containing Glycerol molecules.
Glycerol boasts several striking properties that explain its wide-ranging applications across industries. As it dissolves easily in water-based solutions, glycerol finds many uses across diverse fields and applications. Its extremely high boiling point combined with low toxicity make it suitable for many products; its sweet taste often serves as a sweetener or humectant in pharmaceutical or food applications.

Glycerol is an essential molecule to biological processes and serves an integral part in triglyceride formation and composition, acting as the bonding molecule between three different fatty acid molecules and its counterpart glycerol molecules. Glycerol can be obtained via decomposing triglycerides through metabolism; once decomposed it can either produce energy directly or be transformed into glucose through the Glyceroneogenesis process.
Due to its unique properties and importance in metabolism, Glycerol finds numerous applications in pharmaceuticals, food cosmetics and personal care products, among them pharmaceuticals such as antifreeze. Preservatives that use Glycerol include solvent formulations with preservative properties as moisturizers while viscosity enhancement features can use Glycerol too; its use includes moisturizers in preservative formulations as well as viscosity enhancers used as viscosity enhancers in various formulations formulated using.
Properties of Glycerol
Glycerol (commonly referred to as Glycerin or glycerine) boasts several vital properties that are the cornerstone of its application in various fields.
Here are its primary qualities:
- Physical Status: Glycerol is a colorless viscous, odorless, and odorless liquid at the temperature of room. It has a thick, syrupy consistency and a high viscosity which is why it flows slow.
- Solubility: Glycerol is extremely soluble alcohol, water and a variety of organic solvents. It creates homogeneous, clear solutions when mixed in water and its soluble capacity increases when heated.
- Boiling Point: Glycerol is a liquid with a high boiling point, which is around 289 ° Celsius (554 degree Fahrenheit). This is why it has a high boiling point. it ideal for processes that require high temperatures.
- Melting Point: Glycerol has an approximate melting point of 18 deg Celsius (64 °F). It is able to solidify into crystal form in certain conditions. Typically, this happens at temperatures that are lower than its usual storage and usage temperature.
- Density: Glycerol’s density is about 1.26 grams/cc. It is more dense than water with one gram of density per cubic centimeter. This allows Glycerol to be absorbed by water.
- Taste: A Taste Glycerol is sweet in flavor, like sugar. The sweetness of it makes it a popular ingredient in food products as well as pharmaceutical preparations.
- Hygroscopicity Glycerol is hygroscopic: This means it is able to draw water and absorb it from the surrounding. This is a great humectant that allows it to hold moisture and prevent drying in many products like soaps, cosmetics, and moisturizers.
- Chemical Stability: Glycerol can be used in regular environments to ensure chemical stability. Since it does not suffer degradation or oxidation, glycerol makes an ideal long-term storage and usage solution.
- Non-toxic: Glycerol is generally recognized as safe and non-toxic for both people and animals alike, making it popularly used in food, pharmaceutical cosmetics and personal items without significant side effects or harmful reactions.
These properties make glycerol adaptable and beneficial in a variety of industries, such as food and beverages pharmaceuticals, cosmetics, personal health and chemical manufacturing. The solubility, viscosity as well as its stability and sweet flavor contribute to its wide range of uses and acceptance as a useful compound in a variety of products.
Functions of Glycerol
Glycerol plays an essential role in biological systems and many industries alike.
Here are its primary uses:
- The backbone of Triglycerides: Glycerol serves as the basis of triglycerides; this molecule forms their structure to store oils and fats within living organisms. Triglycerides contain three fatty acid molecules bonded with an additional three molecules made up from glycerol molecules for efficient energy storage compared with proteins or carbohydrate molecules.
- Glycerol is an energy source: Once taken up into metabolic pathways such as glycolysis, glycerol enters glycerol-3 phosphate is then processed by cells into ATP; providing essential fuel when fasting occurs or energy needs increase significantly.
- Glycerol Is an Important Precursor of Biosynthesis: Glycerol plays an essential role in human biosynthesis of different compounds. For instance, glucose production can use this precursor via a process known as Glyceroneogenesis; Glycerol may also be utilized as part of cell membrane formation by producing Phospholipids that play such an essential role. Other biomolecules and essential lipids also rely on it.
- Osmoregulation: Glycerol provides essential osmotic regulation inside cells by helping maintain osmotic equilibrium within them. Glycerol has proven compatible with cell processes and therefore allows accumulation without disrupting processes within. By controlling the osmotic pressure within each cell, glycerol helps prevent excessive loss or gain of water during exposure to various environmental conditions – thus protecting their survival and functionality.
- Humectant and Moisturizer: Glycerol acts as both a humectant and moisturizer; drawing water out from its surroundings while at the same time keeping skin soft and smooth. As such, glycerol makes for an effective component in skincare, cosmetics, and moisturizing products; its properties enable it to draw in water to help hydrate it effectively while simultaneously softening and smoothening it further.
- Preservative and Sweetener: Glycerol is an organic preservative known for preventing the proliferation of microbes, helping extend the shelf-life of foods without spoilage and preventing spoilage altogether. Due to its sweet taste it makes Glycerol an excellent sweetener used both pharmaceutically and food formulations; offering healthy alternatives to sugar.
Understanding glycerol’s function in different fields – nutrition, biochemistry and cosmetics, pharmaceuticals or even food sciences – is fundamental. From its role in biosynthesis and energy metabolism through to osmoregulation and product formulation are major reasons behind its widespread recognition as an indispensable chemical in numerous applications.
Sources of Glycerol
Glycerol can be obtained both synthetic and naturally from sources.
Here are its major sources:
- Vegetable Oils: Vegetable oils like soybean, palm, canola and olive oils contain Glycerol in abundance, triglycerides composed of 3 molecules linked together by Glycerol chains. When broken down through hydrolysis or saponification processes, their Glycerol content becomes the by-product.
- Animal Fats: Animal fats such as lard, tallow and poultry fat contain significant levels of Glycerol found as Triglycerides; similarly with vegetable oils this process often yields Glycerol as the final by-product.
- Biodiesel Production: Glycerol Production for Biodiesel Glycerol is one of the by-products from the biodiesel production process, created as an intermediate product from transesterification of animal fats or vegetable oils using alcohol such as Methanol or Ethanol; Glycerol produced during this step can serve as an essential source for Glycerol.
- Fermentation: Glycerol can be produced through the fermentation of carbohydrates or sugars by microorganisms such as yeast or bacteria, creating organic by-products like glycerol as part of their waste products.
- Industries: Glycerol can also be produced as a by-product from several industrial processes, including soap manufacturing and producing fatty acids or various chemicals.
- Synthetic Production: Glycerol is an organic chemical compound produced through various petroleum-derived processes like propylene or epichlorohydrin production. Industrial applications for synthesized glycerol exist.
- Dietary sources of Glycerol: You can obtain Glycerol by eating certain food products that contain high-fat contents like cheese, butter and meats with fat content – these items provide excellent sources of Glycerol which forms part of triacylglycerides (triglycerides).
Glycerol from any of these sources can be used in diverse applications, from food and beverages production, cosmetics and personal care products production, pharmaceutical processes as well as industrial processes. It should be kept in mind that different sources may produce different quality glycerol levels which require further purification depending on its purpose of use.
What are Fatty Acids?
Fatty acids, an organic compound known as carboxylic acids with four to 36 carbon-atom chains, form the fundamental building blocks for many oils, fats and certain hormones found in nature. Fatty acids serve as primary constituents of biological membranes as well as play important physiological functions within living organisms.
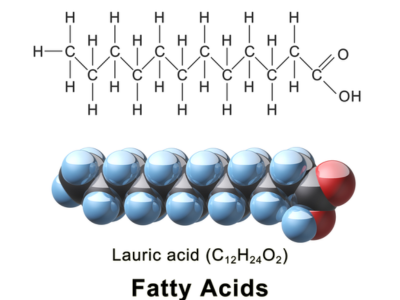
Fatty acids consist of an interwoven hydrocarbon chain known as an acyl chain joined at either end by carboxyl groups (-COOH). Saturation or unsaturation depends upon whether double bonds exist among carbon atoms in this chain of hydrocarbons.
- Saturated Fatty Acids: Saturated fatty acids lack two bonds within their chains of hydrocarbons and instead use hydrogen atoms as filler atoms instead. As such, their linear structures typically remain solid at room temperature; popular examples being palmitic and stearic acids which can be found both in animal fats as well as vegetable oils.
- Unsaturated Fatty Acids: Acids contain two or more double bonds within their hydrocarbon chains, creating flexible or bent structures.
- Monounsaturated Fatty Acids (MUFAs): Have one double bond in their chain of hydrocarbons; an example being Oleic Acid found in canola and olive oils as an example.
- Polyunsaturated Fatty Acids (PUFAs): These essential fatty acids contain two or more double bonds in their chain of hydrocarbons and should be consumed regularly as part of our diet as essential fats for human health.
Fatty acids play several important roles in biological systems:
- Energy Storage: Fatty acids provide our body with energy storage solutions. Once broken down through beta-oxidation, they release significant quantities of ATP which then fuel cells by providing energy production.
- Structural Components: Fatty acids are key building blocks of lipids such as phospholipids, triglycerides and cholesterol esters; as essential parts of cell membranes. Fatty acids aid bilayer formation of these lipids allowing compartments of cells to form.
- Signaling Molecules: Certain fat acids act as signaling molecules or precursors for signal production; for instance arachidonic acid acts as an arachidonate precursor that contributes to producing prostaglandins that aid blood clotting processes among other biological functions.
- Transport and Absorption: Fat acids enter the bloodstream via binding with transport proteins that convert them to lipoproteins like chylomicrons or VLDL – very low density lipoproteins which assist with absorption, transportation and effective delivery to cells throughout the body.
Understanding the significance and classifications of fat acids are integral in comprehending their influence on metabolic, health and various biological processes.
Properties of Fatty Acids
The fatty acids have several significant characteristics that affect their physical and chemical properties.
Here are the main qualities of fatty acids:
- Physical State Physical State: The physical state of fat acids differs based on the length of their chains and the degree of saturation. Short-chain fats (up up to six carbon atoms) generally solid at ambient temperature whereas medium-chain fats (6 -12 carbon atoms) may be fluid or solid. The long-chain acid (more than 12 carbon atoms) tend to be solid at ambient temperature There are exceptions to this rule for certain unsaturated long-chain fat acids that may be liquid.
- Melting Point: The melting points of fatty acids varies based on their chain length as well as the degree of saturation. saturated fatty acids have higher melting points than unsaturated fatty acids with the same length chain. This is due to the saturated fats’ linear structure facilitates tighter packing and more powerful intermolecular forces. Unsaturated fats have lower melting temperatures due to their double bond structure which create kinks in their structure, causing disruption to tight packing.
- Solubility: Fat acids are not soluble in water They are soluble in organic solvents such as chloroform, ether or the ethanol. This is due to the fact that the tail end of hydrocarbons in a acids is nonpolar The carboxyl group at the opposite end is nonpolar. Thus, fatty acids may make hydrogen bonds with Polar molecules, but are more likely to join in nonpolar molecules.
- Hydrophobicity: Fat acids can be described as hydrophobic which means they are repellent or not easily soluble in water. This characteristic is a result of its nonpolar hydrocarbon chain which is incompatible with the polarity of the water molecules. It plays a role in the process of fatty acids when it comes to forming hydrophobic barriers like bilayers of lipids in cell membranes.
- Reactivity: Fatty acids are able to undergo a variety of chemical reactions because of their presence in the carboxyl molecule at the end of their molecule. The carboxyl group is able to participate in esterification reactions and form esters in alcohols, and also in reduction or oxidation reactions. The reaction of fatty acids is dependent on their saturation and unsaturated acids more vulnerable to the process of oxidation.
- Chain Length and Saturation: The length of the chain and its saturation Fatty acids show a vast variety of lengths for chains, ranging from as little as 4 carbon atoms, to up to 36 or even more. Whether double bonds are present or not determine the degree of saturation. Saturated fatty acids lack double bonds, whereas unsaturated acids have two or more double bonds. The length of the chain and the degree of saturation impact the biological and physical properties of fatty acids.
Understanding the functions of the fatty acids are essential for understanding their roles in metabolism, nutrition and the development of lipid structures within living organisms.
Functions of Fatty Acids
Fatty acids serve an array of essential purposes within our biological ecosystems.
Here are their essential uses:
- Energy Storage: Fatty acids provide our bodies with energy through breaking down fat in our diet or stored triglycerides, then transporting these fatty acids directly to cells through beta-oxidation for energy production. When processed within mitochondria, ATP production occurs as energy for each individual cell in our bodies.
- Structural Components: Fatty acids play a vital role in creating structural components in tissues and cells. Phospholipids make up part of cell membranes; Fatty acids play an integral part in maintaining their integrity and fluidity as barrier systems and platforms for cell processes.
- Precursors to Signaling: Molecules Fatty acids serve as precursors for signaling molecules to produce specific signaling reactions in our bodies, Including arachidonic acid an omega-6 polyunsaturated fatty acid that acts as an ingredient to create prostaglandins, thromboxanes and leukotrienes eicosanoids that play key roles in controlling blood clotting, Inflammation and Immune Reactions.
- Protection and Insulation: Fatty acids play a vital role in protecting and insulate organisms. Adipose tissue contains special cells known as adipocytes which store triglycerides derived from fat acids; this insulation acts to regulate body temperature as well as provide organ protection from mechanical shocks.
- Hormone Regulation: Fat acids play an essential part in regulating hormones. Adipose tissue secretes adipokines which contain signals derived from fatty acids derived from leptin, adiponectin and resistin that influence appetite control as well as energy balance, insulin Sensitivity and Inflammation. These signals play a pivotal role.
- Membrane Fluidity and Function: Fatty acids have an enormous influence over the functionality and fluidity of cells’ membranes. Their degree of saturation as well as chain length has an impactful influence; unsaturated fatty acids tend to enhance fluidity while saturated ones decrease it, with unsaturated ones contributing more fluidity while saturated ones decrease it; fluidity is vital to efficient membrane protein transporters and receptors functioning correctly.
- Fatty acids: particularly omega-3 polyunsaturated fatty acids – are vital components in brain cell membranes and play an integral part in neuronal transmission and inflammation control. Fatty acids play an essential role in keeping our minds sharp by providing essential neurotransmitter release as well as inflammation management.
Understanding fatty acid’s function in our lives is of vital importance in order to recognize their significance in cell metabolism, metabolic rate, and overall health. Diet and oil consumption can influence numerous biological processes and increase your risk for chronic illnesses.
Sources of Fatty Acids
Fatty acids can be obtained through food sources as well as synthesizing them within our bodies, with main sources including:
- Dietary Fats and Oils: Fatty acids can be found both animal and plant sources of dietary fats and oils, with some common sources such as margarine, butter and vegetable oils such as olive, canola or soybean oil; as well as poultry meat nuts fish dairy or other products containing them containing various kinds and amounts of saturated monounsaturates polyunsaturates acids found within. The composition may also differ by type.
- Fish and Seafood: Fatty fish such as mackerel, salmon, trout, sardines and others provide essential omega-3 long-chain fats such as EPA and DHA which play an integral role in brain and cardiovascular health, inflammation reduction and overall wellbeing.
- Nuts and Seeds: Nuts and seeds contain various forms of fats that provide essential monounsaturated and polyunsaturated fatty acids for good health, including walnuts, almonds flaxseeds sunflower seeds being excellent examples. All are great food sources of healthy omega-3 and omega-6 fatty acids to give a nutritional boost for daily wellbeing!
- Avocado: An avocado is an exceptional fruit packed with monounsaturated fatty acids – particularly oleic acids – which provide essential nutrition. Thanks to these key components, its smooth texture and nutritional advantages contribute significantly to its success and nutritional advantages.
- Meat and Poultry Products: Animal products like pork, beef lamb and poultry all contain saturated fatty acids in their diet, with specific types depending on breed and species of the animal consumed by consumers.
- Dairy Product: Dairy products like cheese, milk and yogurt contain various amounts of dietary fats such as unsaturated and saturated lipids such as medium-chain and long-chain fats that provide vital nutritional support to our bodies.
- Vegetable Oils: Are One Of Our Main Sources of Fat In the diet, vegetable oils from plant sources such as sunflower seeds, soybeans, canola and corn are our primary source of unsaturated fatty acids; including polyunsaturates and monounsaturates.
- Plant-Based Fats: Coconut oil, palm oil and cocoa butter contain high concentrations of saturated fatty acids while also offering small amounts of polyunsaturated and monounsaturated lipids.
- Synthesis within the Body: The body can synthesize fat acids not consumed through diet; for instance, your liver produces them via lipogenesis (de novo). Unfortunately though, their capacity to synthesize essential fatty acids such as omega-3s and omega-6s are limited, thus making including them in your diet essential.
As important as it is to consume healthy oils and fats in moderation, eating an appropriate balance of various fatty acids along with reviewing one’s overall diet is also key for optimal health and disease prevention. Achieve maximum wellbeing through optimal health through an appropriate balance in nutrition should always be the goal!
Differences between Glycerol and Fatty Acids
Glycerol and fat acids are both key constituents of lipids. Their composition differs substantially –
these being some key distinctions between them:
- Chemical Structure: Glycerol is an alcohol trihydroxy compound composed of three carbons with each carbon carrying its own unihydroxyl (-OH) group; in contrast to this structure fatty acids are carboxylic acids with long hydrocarbon chains ranging between 4 and 36 carbon atoms and often have more complex structures with numerous hydrogen groups than that found in Glycerol or its close cousin Glycerin.
- Composition: Glycerol can be defined as an amalgamation of carbon, hydrogen and oxygen molecules; its molecular formula being C3H8O3. Fatty acids likewise consist of hydrogen atoms with their molecular formula depending on length of chain and saturation level.
- Role in Lipids: Glycerol serves a critical role in lipids production: as the backbone for synthesizing triglycerides, phospholipids, and glycerophospholipids. Triglycerides consist of three fatty acid molecules esterified to the hydroxyl group in Glycerol that create an esterification reaction with its ester group to form one long glycerol chain bearing three tails attached by 3 esterified esters; Glycerophospholipids/ glycerophospholipids contain two chains of fatty acid chains joined to Glycerol’s third group of hydroxyls as part of their makeup;
- Physical Properties: Glycerol is an innocuous colorless viscous substance with hydrophilic qualities; that means it absorbs and attracts water from its surroundings, such as rain. It has an appealing taste and boiling point of around 290degC (554degF). Fats may turn solid when heated to room temperature depending on their length of chain or saturation levels – saturated fatty acids typically solidifying while unsaturated ones might turn liquid or vice versa.
- Functional: Glycerol plays an essential part of energy metabolism by serving both as an intermediate in the glycolysis pathway and through its conversion to sugar pyruvate or glucose for energy production, respectively. Fatty acids provide another source of energy production within our bodies via beta-oxidation; their metabolism results in producing ATP for use as energy storage in cells.
- Solubility: Glycerol is highly water soluble due to the hydroxyl group’s ability to form hydrogen bonds with water molecules; in comparison, fat acids tend to be hydrophobic and insoluble within water; they dissolve best with organic solvents like chloroform or ether.
- Biological Function: Glycerol plays an essential role in various physiological processes, from energy storage and storage osmoregulation, through production of complex lipids to acting as cryoprotectants in certain organisms that protects them against freezing damage to cell structures when frozen. Fatty acids play an integral part in cell membrane structure regulation as well as energy storage along signaling and communication pathways.
Glycerol and fatty acids each possess specific structures, functions and characteristics in biological systems that make them useful components. Glycerol plays an essential part in synthesizing lipids while fat acids serve as primary constituents. Together these lipids offer support, energy provision and even regulatory functions within organisms and cells.
Importance of Glycerol and Fatty Acids
Fatty acids and glycerol play an integral part in biological systems, here are their top benefits:
Glycerol plays an essential role:
- Energie Metabolism: Glycerol is an integral element in energy metabolism. By breaking down triglycerides, its molecules are released and converted to glucose or even pyruvate via an organic pathway known as the gluconeogenesis process – with these then entering pathways producing energy to eventually generate ATP; which serves as the main currency within cells.
- Lipid Synthesis: Glycerol is an indispensable ingredient when producing various liquids and serving as the basis of triglyceride synthesis – they store fat in our diet’s adipose tissue as triglycerides; additionally they’re an efficient form of concentrated energy used when energy demands spike significantly.
- Membrane Formation: Glycerol is an indispensable ingredient in producing phospholipids – key constituents of cell membranes – through production. Phospholipids feature an inverted pyramid structure made up of backbone composed of two fatty acid chains linked together by an ester linkage and the phosphate group; together these form bilayer lipids which structure cell membranes. Glycerol’s presence promotes membrane flexibility and dynamic behavior so as to perform crucial functions, such as selective permeability or communication between cells.
Fatty acids’ importance:
- Energy Source: Fatty acids provide energy sources to the body in many different forms. Food-derived or stored triglycerides break into smaller pieces to release these fatty acids which then undergo beta-oxidation before eventually becoming transformed into ATP for energy use during physical activities or fasting periods. Fatty acids provide powerful energy reserves which provide efficient sources of sustained power during prolonged physical activities or fasting periods.
- Structure Component: Fatty acids play a fundamental role in biological systems and processes, from their essential role as key constituents of cell membranes to transportation, signaling, and intercellular interactions. Fatty acids serve an indispensable purpose and play an integral part in keeping biological processes moving along smoothly and functioning correctly. Phospholipids make up this bilayer of lipids which forms cell membranes while Fatty acids play their part by controlling membrane permeability, fluidity and stability allowing cell membranes to remain intact for cell transport, signaling intercellular interactions as well as inter cell interactions among cells themselves.
- Hormone Regulation: Certain fatty acids act as precursors in the production of signaling molecules called eicosanoids, such as prostaglandins, leukotrienes, and thromboxanes that play an integral part in controlling blood clotting, inflammation, immune responses and biological processes. Omega-3 as well as omega-6 polyunsaturated fat acids play an essential part in this production of these eicosanoids.
- Nutrient Absorption: Fat acids play an integral part in absorbing and transporting fat-soluble vitamins such as A, D, E and K as well as other lipid-soluble substances through digestion and absorption in the small intestine, where they become chylomicrons – lipoproteins which transport these substances through circulation and lymphatic systems – for transport back out again into our bodies.
- Brain Function: Fatty acids, particularly omega-3 polyunsatured fatty acids like docosahexaenoic acid (DHA), play an integral part in brain development and functioning. DHA forms part of its cell membranes and plays an essential role in neuronal signalling, plasticity and overall cognitive wellbeing.
Fatty acids and glycerol play an integral part in various physiological processes that support life, from energy metabolism and cell structure/function maintenance, hormonal regulation of absorption of nutrients as well as brain functioning. Their essentiality goes far beyond energy storage as these vital compounds form part of vital biological functions within organisms.
Summary and Conclusion
Fatty acids and glycerol are two crucial elements of biological systems, playing key roles as energy providers as part of metabolic processes involving lipids; providing energy, participating in metabolism processes related to these compounds as well as contributing to cell membrane structure. Meanwhile, fatty acids serve a similar role within cell membrane structures by providing energy as structural elements as well as acting as hormonal regulators by helping with absorption and functioning as neuroprotectants regulating hormone production while aiding brain functionality.
Glycerol and fatty acids play a vital role in energy metabolism as their breakdown is used to generate ATP – the main currency used by cells. Both act as precursors in creating various oils like triglycerides and phospholipids which store energy, act as insulation material in cell membranes, as well as ensure integrity within them. Fat acids also play a precursor role for signaling molecules such as eicosanoids which regulate inflammation as well as other biological processes; additionally, they both play key functions during absorption of nutrients as well as helping with brain development and function overall.
Knowledge of fatty acids and glycerol’s functions, properties, sources and distinctions will give us insight into their significance within biological systems. They play a pivotal role in energy metabolism as well as cell structure maintenance, signal control and overall wellbeing improvements – vital elements that contribute to overall wellbeing!