L and D Amino Acids
The difference between L and D amino acids is that L is the isomer of an amino that can rotate plane-polarized lights anticlockwise. The amino acid is a simple organic molecule that has a basic amino group, a carboxyl acid group (-COOH), and a variable “R” group attached to a central sp3 hybridized carbon atom.
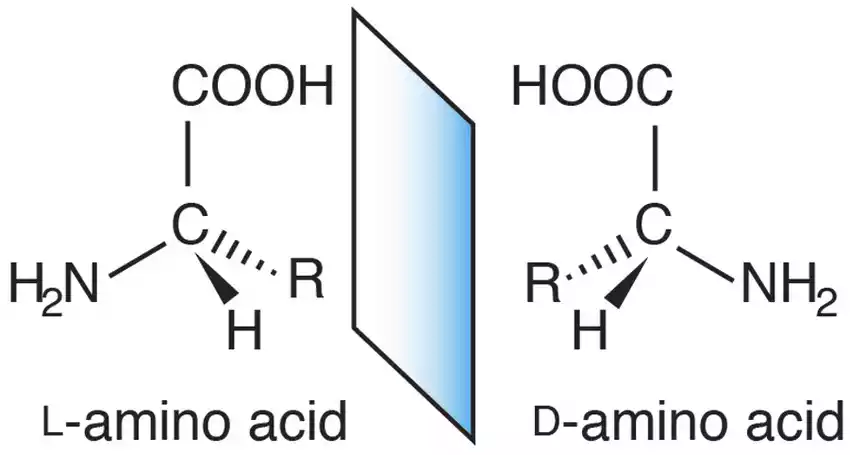
The R group can have different properties and the wide range of possible chemical groups that can bind to central carbon atoms as R groups gives amino acids a huge variety of versatility. Amino acid subunits are known for their structural properties. They make up proteins. They are also intermediates in cellular metabolism.
What is Chirality?
The chirality of a particular organic compound results from the presence of chiral Carbons in that molecule. All alpha-amino acids – with the exception of glycine, which has two hydrogen atoms that are indistinguishable and bound to its alpha carbon- have chiral carbons. The chiral carbons in alpha-amino acids allow stereoisomerism.
As a result, all alpha amino acids except glycine are able to form two stereoisomers, mirror images. These mirror images are called enantiomers and they are named ‘L’, ‘D’ or a combination of both (L/D nomenclature). This enantiomeric distinction is important for biological reasons, regardless of its nomenclature. It occurs because amino acids interact only with molecules that are sensitive enough to recognize one of two possible enantiomers.
What is L Amino Acids?
The L amino acid, when dissolved in water, causes the plane-polarized spectrum to rotate anticlockwise. The Latin word “Laevus” means “left”. The polarimeter is used to measure this rotation, which is called “optical activity”. In spite of the fact that both L and D amino acids exist, it is surprising to find only L amino acids in most physiological proteins. As a result, many amino acids exhibit an L-enantiomeric surplus in biological systems.
What is D Amino Acids?
D-amino acids are the enantiomers of certain amino acids that can rotate plane polarized lights clockwise. These enantiomers, which incorporate the Latin word “Dexter” – meaning “right”, are called D-enantiomers. D-amino acid is not normally produced by cells and incorporated into protein. Some D-amino acid can be found within bacterial cell wall, but not bacterial protein.
D-amino acids are not common in biological systems but they play a vital role in many situations. Racemase enzymes of Vibro cholera, for example, are responsible for the production of D-forms of leucine and methionine during slow growth. This reduces the production of peptidoglycans.
Difference Between L and D Amino Acids
L- and D-amino acids differ primarily in terms of their stereochemistry – that is, how their functional groups arrange around an alpha carbon.
Here are some main distinctions between D and L amino acids:
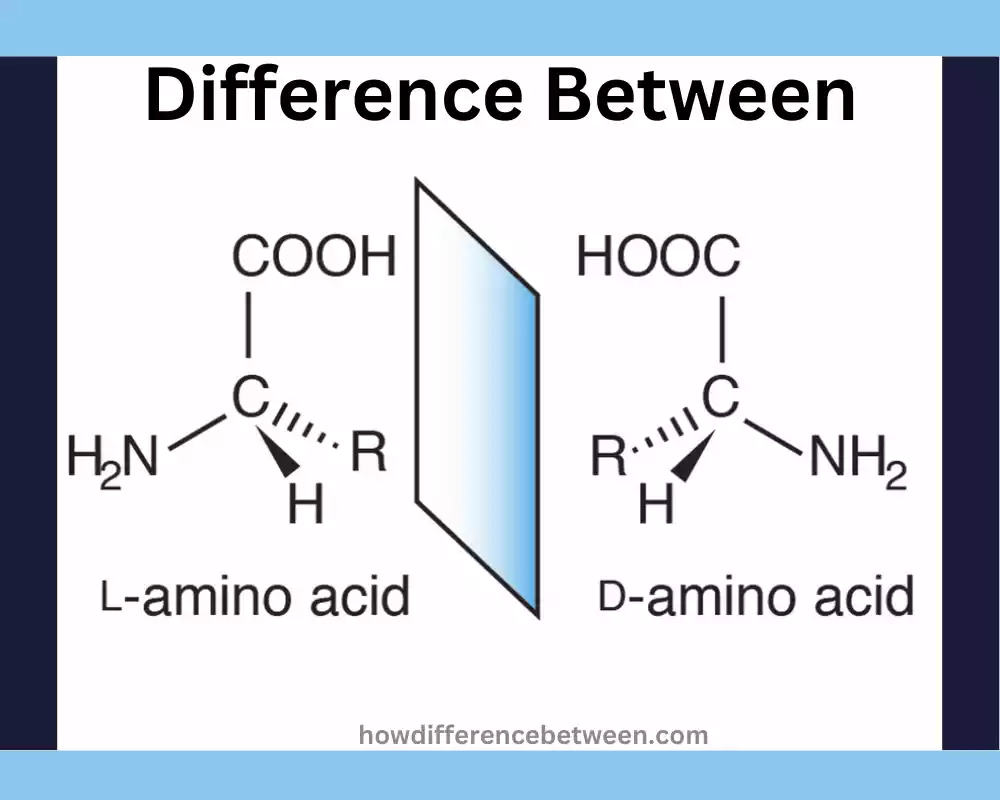
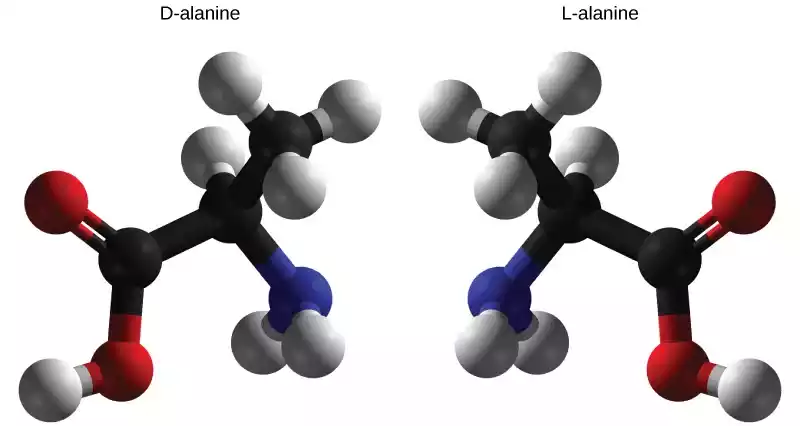
- Configuration: Fischer projection shows the amino group of L-amino acid (-NH2) located to the left of its central alpha carbon; D-amino acid has its amino group on the right of this central carbon; thus their designations, L and D respectively, refers to differences in their structures.
- Mirror Images: L and D amino acids, also referred to as enantiomers, are mirror images, similar to your left and right hands which cannot be placed over each other. D-amino acid occurs less commonly but still exists within some organisms, non-protein molecules and peptides.
- Enzymatic Selectivity: Enzymes in living systems generally preferentially recognize L-amino acid for substrate binding and catalysis, making L-amino acid an integral part of their active sites and contributing significantly to specificity and efficiency of enzyme reactions. This selectivity plays a pivotal role in specificity and efficiency.
- Protein Structure: Most living organisms’ proteins consist largely of L amino acids. Their sequence determines their function and three-dimensional structure; even one D amino acid may significantly change this.
- Biological Significance: Their use is crucial to many biological processes like cell signaling and structural support as well as enzyme reaction. D-amino acid may have less common biological roles such as cell wall formation.
Assigning an amino acid with either L or D suffices does not equate to biological significance and activity; stereochemistry plays a crucial role in how amino acids interact with enzymes, receptors and other biomolecules present within living systems – ultimately adding value and significance in terms of biological processes and ecosystem services provided.
Similarities of L and D Amino Acids
L and D amino acids are mirror-image isomers of each other, meaning they share certain similarities despite their opposite spatial arrangements around the chiral α-carbon.
Here are some similarities between L and D amino acids:
- Chemical Structure: L and D amino acids have the same chemical formula and basic structure. They both consist of an α-carbon bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and an R-group (side chain).
- Functional Groups: The functional groups present in L and D amino acids are identical. Both contain amino and carboxyl groups, which are key components involved in peptide bond formation during protein synthesis.
- Chirality: Both L and D amino acids are chiral molecules, meaning they have non-superimposable mirror images. This chirality arises from the presence of the α-carbon with four different substituents.
- Enantiomers: L and D amino acids are enantiomers of each other, which means they are stereoisomers that are mirror images. Enantiomers have identical physical properties (such as melting and boiling points) but interact differently with plane-polarized light.
- Biological Building Blocks: Both L and D amino acids are used as building blocks for biological molecules. While L-amino acids are the primary constituents of proteins in living organisms, some D-amino acids are found in certain natural systems, such as bacterial cell walls and peptides.
- Peptide Bond Formation: Both L and D amino acids participate in peptide bond formation during protein synthesis. The reaction involves the condensation of the carboxyl group of one amino acid with the amino group of another, resulting in the release of water and the formation of a peptide bond.
- Amino Acid Properties: Many of the general chemical and physical properties of L and D amino acids are the same, such as their acid-base behavior, reactivity, and functional group interactions.
It’s important to note that while L and D amino acids share these similarities, their differing spatial arrangements have profound effects on their biological interactions and functions. In living organisms, L-amino acids are overwhelmingly favored due to their prevalence in proteins and their compatibility with biological processes. D-amino acids, while less common, can have specific roles in certain biological contexts.
Ending
The symbiotic relationship between L and D Amino Acids underscores the marvels of nature’s precision. Their harmonious interplay, though often unseen, orchestrates the symphony of life, reminding us of the profound intricacies that govern our existence. As we continue to unravel their secrets, we embark on a journey towards deeper understanding and endless possibilities.