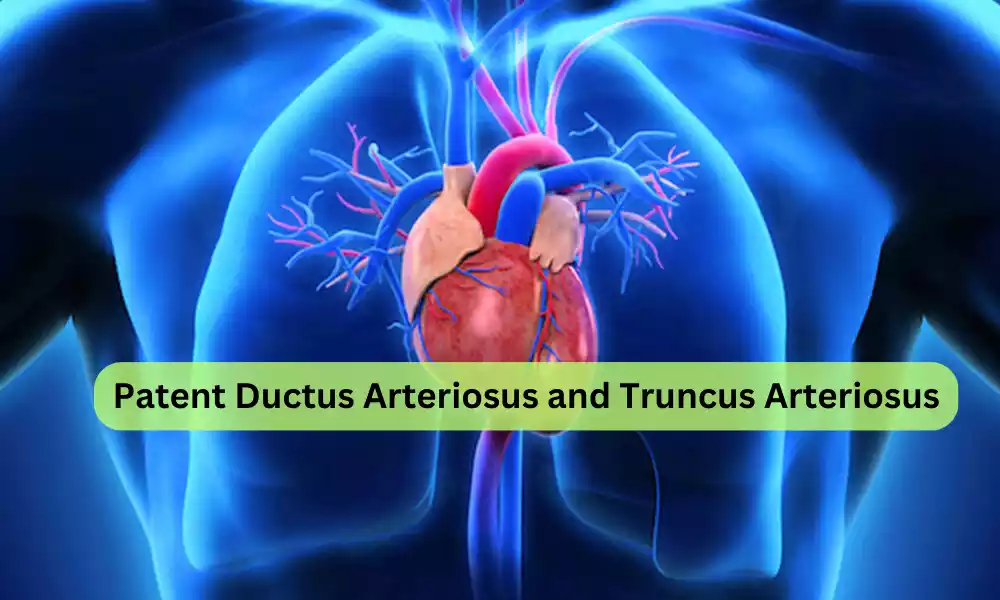
Patent Ductus Arteriosus and Truncus Arteriosus are congenital heart conditions that arise from structural abnormalities in the cardiovascular system before birth. PDA involves an abnormal persistence of the ductus arteriosus, a vessel that ordinarily closes after birth, resulting in an aberrant connection between the aorta and pulmonary artery. On the other hand, Truncus Arteriosus is characterized by a single common vessel emerging from the heart instead of the typical two distinct vessels, often accompanied by a ventricular septal defect.
Both these anomalies disrupt the normal flow and oxygenation of blood, requiring precise diagnosis and timely interventions to prevent life-threatening complications and improve the long-term prognosis for affected infants.
What is Patent Ductus Arteriosus?
Patent Ductus Arteriosus (PDA) is a congenital heart defect wherein the ductus arteriosus, a fetal blood vessel that connects the main pulmonary artery to the aorta, fails to close after birth. In a typical fetal circulation, the ductus arteriosus allows blood to bypass the lungs since the fetus gets its oxygen from the placenta. However, shortly after birth, this vessel is supposed to close as part of the natural transition from fetal to postnatal circulation.
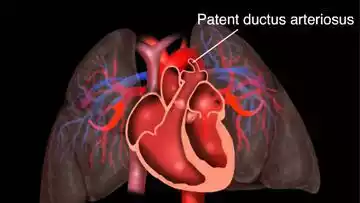
When it remains open (or “patent”), it can cause oxygen-rich blood from the aorta to mix with oxygen-poor blood from the pulmonary artery. This can lead to increased workload on the heart and increased blood flow to the lungs.
Causes and risk factors
The exact cause of PDA is not always clear, but several factors have been associated with an increased risk of having this congenital defect:
-
- Prematurity: PDA is more common in premature infants. The ductus arteriosus tends to close within the first couple of days after birth in full-term infants, but it might remain open in premature infants.
- Family History and Genetics: If there are other family members with PDA or other congenital heart defects, the risk of having PDA increases. Certain genetic conditions, such as Down syndrome, are also associated with a higher risk of PDA.
- Rubella Infection During Pregnancy: Mothers who contract the rubella virus (German measles) during pregnancy have an increased risk of giving birth to a baby with PDA.
- Other Maternal Health Conditions: Diabetes in the mother, if not well controlled, can increase the risk of the baby having PDA.
- Birth at High Altitudes: Infants born at high altitudes (above 10,000 feet) have a higher risk of developing PDA, potentially due to the decreased oxygen levels at these altitudes.
- Sex: Females are somewhat more likely than males to experience PDA.
- Other Congenital Disorders: Babies with certain congenital disorders, such as Down syndrome or a congenital diaphragmatic hernia, may have an increased risk of PDA.
- Multiple Births: Being a twin, triplet, etc., especially if one of the co-twins dies either before or shortly after birth, can increase the risk of PDA.
While these factors may increase the risk of having PDA, they don’t directly cause it. Many babies with PDA have no identifiable risk factors. Additionally, having one or more of these risk factors doesn’t guarantee that a baby will have PDA.
Symptoms of PDA
The symptoms of PDA can vary based on the size of the ductus and the amount of blood flow it allows. While small PDAs might not cause any noticeable symptoms, larger PDAs can lead to significant problems.
Here are the typical symptoms and signs associated with PDA:
- Heart Murmur: This is often the first sign of PDA. A heart murmur is an unusual sound heard during a heartbeat. Specifically, the murmur of PDA is often described as a “machinery” murmur because of its characteristic sound.
- Tachycardia: Faster than normal heart rate.
- Respiratory Symptoms:
- Rapid breathing or breathlessness
- Increased susceptibility to respiratory infections
- Wheezing
- Poor Feeding and Growth:
- Poor feeding habits, especially in infants
- Difficulty in gaining weight or slower growth patterns
- Fatigue or Tiredness: Due to the heart working harder, a person may become easily fatigued or tired, especially during activities.
- Sweating: Excessive sweating, especially during feedings in infants.
- Bounding or Strong Pulses: Because of the increased flow of blood, pulses may feel stronger than usual.
- Cyanosis: A blue or dusky skin color, which indicates decreased oxygen in the blood. This symptom is less common and usually indicates a large PDA with significant shunting of blood.
- Enlarged Heart: As the heart works harder to pump blood, it can become enlarged over time. This might not be an immediate symptom but can be a consequence of untreated PDA.
- Symptoms of Heart Failure:
- Swelling or puffiness (edema) of the legs, ankles, and feet
- Shortness of breath, especially when lying down
- Fatigue and weakness
It’s essential to remember that not all individuals with PDA will exhibit all these symptoms. Some might have very mild or no symptoms, especially if the PDA is small. However, even in the absence of symptoms, PDA can have long-term consequences on the heart if not addressed. Regular check-ups with a pediatrician or cardiologist can help monitor and manage the condition appropriately.
Diagnosis methods
Early detection and diagnosis of PDA are crucial for appropriate management and prevention of potential complications. Here are the common diagnostic methods used to identify and assess PDA:
- Physical Examination:
- Heart Murmur: Often, the first indication of PDA is the detection of a characteristic “machinery” murmur during a routine physical examination using a stethoscope.
- Other Findings: Signs like bounding pulses or symptoms of heart failure might be noted during a physical check.
- Chest X-ray:
- Can provide images of the heart and lungs.
- Might show an enlarged heart or increased fluid or blood flow to the lungs, suggesting PDA.
- Echocardiogram (Echo):
- Primary diagnostic tool for PDA.
- Uses sound waves to produce images of the heart, allowing visualization of the heart’s structures, including the ductus arteriosus, and assessing the blood flow through the heart.
- Can measure the size of the PDA and determine the amount of blood flow it’s allowing.
- Electrocardiogram (ECG or EKG):
- Measures the electrical activity of the heart.
- Can show patterns suggestive of strain or enlargement of certain heart chambers due to PDA.
- Cardiac Catheterization:
- A more invasive test where a thin, flexible tube (catheter) is inserted into a blood vessel (usually in the groin) and threaded up to the heart.
- Allows direct measurement of pressures in the heart chambers and can provide detailed information about the heart’s structure and function.
- Not commonly used just for diagnosis, but can be employed for therapeutic interventions, like closing the PDA.
- Pulse Oximetry:
- Measures the oxygen saturation in the blood.
- While not specific to PDA, decreased oxygen saturation might raise suspicion, especially in newborns.
- MRI (Magnetic Resonance Imaging) or CT (Computed Tomography) Scan:
- These imaging modalities can provide detailed pictures of the heart and surrounding structures.
- Less commonly used for PDA diagnosis but might be employed in certain situations or for pre-surgical planning.
The selection of diagnostic methods often depends on the patient’s age, clinical presentation, and the expertise and facilities available at the healthcare center. Typically, a combination of clinical examination and echocardiography is sufficient to diagnose PDA in most cases.
Treatment and management
The treatment approach for PDA depends on the size of the ductus arteriosus, the age of the patient, and the presence or absence of symptoms. Here are the common treatments and management strategies:
- Watchful Waiting:
- Small PDAs that don’t produce symptoms might not require active treatment initially. They can be monitored to see if they close on their own, especially in premature infants where spontaneous closure is more common.
- Medications:
- Indomethacin or Ibuprofen: These are nonsteroidal anti-inflammatory drugs (NSAIDs) that can stimulate the ductus arteriosus to close. They are especially used in premature babies.
- Diuretics: Might be used if there are symptoms of heart failure to remove excess fluid from the body.
- Inotropic Agents: Drugs like digoxin might be prescribed to strengthen the heart’s contractions if there’s heart failure.
- Catheter-based Procedures:
- Coil or Device Closure: In this procedure, a catheter is inserted into a blood vessel and threaded to the heart. A coil or a small device is then placed to block the PDA. This method is often preferred for older children and adults.
- Balloon Angioplasty: Rarely used for PDA, but involves inflating a small balloon inside the PDA to widen it, followed by placement of a device to seal it.
- Surgical Closure:
- If the PDA is large or can’t be closed using catheter-based procedures, surgery might be required.
- Ligation: The most common surgical procedure where the ductus arteriosus is tied off or clipped.
- Video-Assisted Thoracoscopic Surgery (VATS): A minimally invasive procedure where small incisions are made in the chest, and instruments are inserted to close the PDA.
- Regular Monitoring:
- Even after treatment, regular check-ups with a cardiologist are essential to ensure that there are no complications and the heart is functioning well.
- Monitoring may include physical exams, echocardiograms, and other tests as deemed necessary by the physician.
- Lifestyle and Ongoing Care:
- Some individuals might need to take precautions before dental procedures or surgeries to prevent infections like endocarditis.
- A heart-healthy lifestyle is encouraged, including a balanced diet, regular exercise, avoiding tobacco, and regular medical check-ups.
The choice of treatment modality often depends on individual circumstances. It’s crucial for patients and caregivers to work closely with their healthcare providers to determine the most appropriate approach.
What is Truncus Arteriosus?
Truncus Arteriosus is a rare congenital heart defect in which a single blood vessel (trunk) emerges from the heart, instead of the usual two separate vessels (the aorta and the pulmonary artery). This single vessel then branches out, supplying blood to both the systemic circulation (to the body) and the pulmonary circulation (to the lungs).
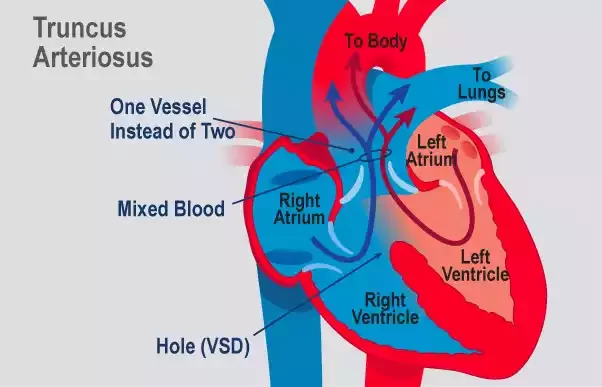
As a result, oxygen-rich and oxygen-poor blood mix together, causing the heart to work harder and leading to reduced oxygen delivery to the body’s tissues. If left untreated, Truncus Arteriosus can lead to serious health complications, including heart failure.
Causes and Manifestations
Causes:
Truncus arteriosus is a congenital (present at birth) heart defect, and its exact cause is not fully understood. Certain factors are associated with an increased risk:
- Genetic Factors: Some children with truncus arteriosus have chromosomal abnormalities, suggesting a genetic component to the condition.
- Environmental Factors: There is some evidence that exposure to certain environmental factors during pregnancy, like certain medications, infections, or chemicals, may increase the risk of having a child with a congenital heart defect, including truncus arteriosus.
- Maternal Conditions: Conditions like poorly controlled diabetes or rubella infection during pregnancy might increase the risk of congenital heart defects.
- Family History: A family history of heart defects can increase the risk, though the direct inherited pattern for truncus arteriosus is not clear.
Manifestations (Symptoms and Signs):
The manifestations of truncus arteriosus are due to the mixing of oxygen-rich and oxygen-poor blood, leading to reduced oxygen delivery to the body’s tissues and overloading the lungs with blood. Common symptoms and signs include:
- Blue or Pale Gray Skin Color (Cyanosis): This results from a reduced amount of oxygen in the bloodstream.
- Shortness of Breath or Rapid Breathing: Due to the excessive flow of blood into the lungs.
- Poor Feeding: Infants may become tired quickly when feeding or may not be able to eat enough to sustain growth.
- Failure to Gain Weight or Grow at the Expected Rate: Due to reduced calorie intake and the increased energy expenditure of the overworking heart.
- Tiring Easily or Becoming Fatigued Quickly During Physical Activity.
- Delayed Development: Children may reach developmental milestones like sitting, crawling, and walking later than their peers.
- Heart Murmur: An unusual sound heard during a heartbeat.
- Excessive Sweating, especially during feedings or exertion.
- Frequent Respiratory Infections: Due to congestion in the lungs.
- Signs of Heart Failure: Such as swelling in the legs, ankles, and feet or fluid retention in other parts of the body.
Since truncus arteriosus is a severe condition, symptoms typically become evident soon after birth. Early diagnosis and appropriate management are crucial for the best outcomes. If parents or caregivers notice any of the mentioned symptoms in their child, they should seek medical attention immediately.
Diagnostic procedures
For diagnosing Truncus Arteriosus, a combination of clinical examination and specialized tests are employed to confirm the diagnosis, assess the severity, and plan appropriate interventions. Here are the primary diagnostic procedures:
- Physical Examination:
- Heart Murmur: During a routine check, the doctor might hear an abnormal heart sound (murmur) using a stethoscope.
- Signs of Cyanosis: Blue or grayish skin coloration, particularly around the lips and fingertips.
- Evidence of Heart Failure: Such as swelling in the ankles, feet, or around the eyes.
- Echocardiogram (Echo):
- This is the primary diagnostic tool for truncus arteriosus.
- It uses sound waves to create detailed images of the heart.
- The echo can show the structure of the heart, the presence of the single large vessel (truncus arteriosus), and the function of the heart’s chambers and valves.
- Electrocardiogram (ECG or EKG):
- This test records the electrical activity of the heart.
- It can identify abnormal heart rhythms and provide insights into heart muscle function.
- Chest X-ray:
- Provides images of the heart and lungs.
- An enlarged heart, a characteristic feature of many congenital heart diseases including truncus arteriosus, can be seen on an X-ray.
- It can also show changes in the lungs due to increased blood flow.
- Cardiac Catheterization:
- A thin, flexible tube (catheter) is inserted into a blood vessel, usually in the groin, and guided to the heart.
- It allows measurement of pressures within the heart’s chambers and the blood vessels.
- Can also be used to visualize the structure of the heart and blood flow patterns using dye injection.
- While not always necessary for diagnosis, it may be used for planning surgical interventions.
- Pulse Oximetry:
- A non-invasive test that measures the oxygen saturation level in the blood.
- Lower than normal oxygen levels may indicate the presence of a heart defect like truncus arteriosus.
- MRI (Magnetic Resonance Imaging) or CT (Computed Tomography) Scan:
- These advanced imaging techniques can provide detailed pictures of the heart and surrounding structures.
- While not first-line diagnostic tools for truncus arteriosus, they might be used in certain situations or for surgical planning.
Once diagnosed, a comprehensive treatment plan is developed based on the severity of the condition, the age of the patient, and other associated anomalies. It’s essential to work closely with a pediatric cardiologist or a cardiac surgeon who specializes in congenital heart diseases.
Treatment options
Treatment for Truncus Arteriosus is primarily surgical, as the condition doesn’t resolve on its own and can lead to severe complications if left untreated. The main goal is to separate the single large vessel into two distinct vessels (aorta and pulmonary artery) and close the ventricular septal defect (VSD).
Here are the primary treatment options:
- Surgical Repair:
- Typically performed within the first few weeks to months of life.
- The surgery involves:
- Closure of the VSD: Using a patch to prevent oxygen-rich and oxygen-poor blood from mixing.
- Separation of the Truncus: The common vessel is divided into two separate arteries – the aorta (which carries blood to the body) and the pulmonary artery (which carries blood to the lungs).
- Creation of a New Pathway for Blood to Reach the Lungs: Since the native pulmonary arteries are often small or underdeveloped, a conduit (tube) with a valve is placed between the right ventricle and the pulmonary arteries. This conduit acts as a new path for blood to flow to the lungs.
- Over time, as the child grows, the conduit may need to be replaced due to outgrowing it or conduit degeneration. This would require additional surgeries.
- Medications:
- Before Surgery: Medications like diuretics (to remove excess fluid from the body), inotropic agents (to help the heart contract better), and other heart failure medications might be used to manage symptoms and stabilize the infant before surgery.
- After Surgery: Antibiotics may be prescribed to prevent endocarditis (an infection of the inner lining of the heart). Blood pressure medications or other heart drugs might be needed depending on the post-operative course.
- Regular Monitoring and Follow-up:
- Even after successful surgical repair, regular check-ups with a cardiologist are essential. These visits often involve physical examinations, echocardiograms, ECGs, and other tests to monitor heart function, the status of the conduit, and check for potential complications.
- Over the course of a lifetime, additional interventions or surgeries might be needed, especially to replace the conduit as the child grows or if the conduit becomes narrowed or its valve becomes leaky.
- Activity Restrictions and Lifestyle Recommendations:
- After recovery from surgery, most children can participate in normal or near-normal activities. However, specific activity restrictions might be advised by the cardiologist based on the individual’s condition and the presence of any complications.
- Maintaining a heart-healthy lifestyle, including regular medical check-ups, is essential.
- Precautions might be necessary before dental procedures or surgeries to prevent infections like endocarditis.
- Interventional Cardiac Catheterization:
- In some cases, if the conduit or pulmonary arteries become narrowed, balloon angioplasty or stent placement might be done via catheterization to widen the narrowed area without open-heart surgery.
Early diagnosis and timely surgical intervention are crucial for improving outcomes and survival rates in children with truncus arteriosus. With proper care and monitoring, many individuals with the condition can lead fulfilling lives. However, it’s essential for them to remain under the care of a cardiologist who specializes in congenital heart diseases throughout their lives.
Comparison Table of Patent Ductus Arteriosus and Truncus Arteriosus
Here’s a comparison table highlighting the main differences between Patent Ductus Arteriosus (PDA) and Truncus Arteriosus:
| Feature/Aspect | Patent Ductus Arteriosus (PDA) | Truncus Arteriosus |
|---|---|---|
| Definition | A persistent opening between the aorta and the pulmonary artery, which normally closes shortly after birth. | A congenital defect where there’s a single large vessel instead of the usual two separate vessels (aorta and pulmonary artery). It also involves a ventricular septal defect (VSD). |
| Causes & Risk Factors | – Prematurity
– Rubella infection during pregnancy – Genetic factors – High-altitude pregnancies |
– Genetic factors
– Environmental factors during pregnancy – Maternal conditions like diabetes – Family history |
| Manifestations | – Mild cases might be asymptomatic
– Heart murmur – Rapid breathing – Increased susceptibility to infections – Poor growth |
– Cyanosis
– Heart murmur – Rapid breathing – Poor feeding – Failure to thrive – Excessive sweating – Frequent respiratory infections |
| Diagnostic Procedures | – Echocardiogram
– Chest X-ray – ECG – Cardiac catheterization (less common) |
– Echocardiogram
– ECG – Chest X-ray – Cardiac catheterization – Pulse oximetry – MRI or CT scan (in specific cases) |
| Treatment Options | – Medications (e.g., NSAIDs)
– Catheter-based procedures – Surgical ligation or closure – Monitoring for smaller PDAs that might close on their own |
– Surgical repair (to separate the single vessel and close the VSD)
– Medications for heart failure symptoms – Regular monitoring and follow-up – Possible conduit replacement surgeries as the child grows |
This table provides a succinct comparison, but each condition has its nuances and complexities. For a comprehensive understanding, further reading or consultation with a cardiologist is recommended.
What are the similarities between Patent Ductus Arteriosus and Truncus Arteriosus?
Here are the similarities between Patent Ductus Arteriosus (PDA) and Truncus Arteriosus presented in bullet points:
- Congenital Origin: Both are congenital heart defects present at birth.
- Abnormal Blood Flow: Both conditions lead to the mixing of oxygenated and deoxygenated blood.
- Increased Heart Strain: Both can cause the heart to work harder, potentially leading to heart failure.
- Common Symptoms: Shared symptoms include rapid breathing, fatigue, poor feeding, and failure to thrive.
- Heart Murmurs: Both conditions can produce detectable heart murmurs during a physical examination.
- Diagnostic Measures: Echocardiography is key for diagnosing both conditions, and other tools like ECG and chest X-rays may also be used.
- Need for Intervention: Both typically require medical or surgical intervention to correct or manage the condition.
- Long-term Monitoring: Individuals with either condition usually require ongoing cardiological follow-up.
- Risk Factors Overlap: Both have associated genetic and environmental risk factors, including certain maternal conditions or exposures during pregnancy.
It’s essential to approach each condition with a thorough understanding, as while they share similarities, their differences are crucial for proper management and care.
Conclusion
Patent Ductus Arteriosus (PDA) and Truncus Arteriosus are congenital heart defects that manifest due to the abnormal formation of the heart’s structures during fetal development. PDA involves a persistent opening in the ductus arteriosus, while Truncus Arteriosus is characterized by a single common blood vessel emerging from the heart.
While their causes remain a blend of genetics and other factors, early detection and medical interventions can significantly improve outcomes, allowing many individuals to lead fulfilling lives. Regular medical check-ups and awareness are vital to manage these conditions effectively.