Overview of RNASE A and RNASE H
RNASE A and H are two different enzymes with distinct roles within cell biology processes.
RNASE A:
RNASE A is an endoribonuclease enzyme found in various organisms and mammals alike that specifically degrades RNA molecules. RNASE A has been widely studied due to its well-documented role in cell turnover processes involving degradation or turnover of RNA molecules; specifically targeting sequences or structures within them which trigger its activity to break phosphodiester bonds and fragment RNA molecules into smaller fragments, helping maintain stable gene expression levels, control gene stability, eliminate aberrant or unnecessary molecules.
While hosting defense immunity by degrading foreign or viral RNA molecules from entering host defense/immune responses against infection by degrading foreign or immunity as well as home defense/immune response mechanisms.
RNASE H:
RNASE H is an endonuclease that specifically breaks apart RNA-DNA hybrids and can be found in both prokaryotic cells as well as eukaryotes. As opposed to its more famous cousin RNASE A, which targets such hybrids formed during specific cellular processes like DNA replication/repair; instead, RNASE H’s main role lies in eliminating primer RNA formed during DNA repair/replication events.
RNASE H is an enzyme that specifically recognizes and degrades RNA strands present in an RNA-DNA hybrid molecule, catalyzing their separation and permitting DNA polymerase or repair enzymes to fill any resulting gaps with DNA polymerase or repair enzymes. As well as serving DNA replication and repair purposes, RNASE H plays a vital role in retroviral replication as retroviruses use their genome RNA as templates for reverse transcription.
During which process RNASE H plays an instrumental part in degrading the RNA strand present during reverse transcription – acting to degrade an RNA-DNA hybrid during reverse transcription process using retroviral genome templates as templates RNA genome templates which retroviruses use their genome as templates while degrading any associated hybridized RNA/DNA hybrid during retroviral replication process RNA-DNA hybridization plays an integral role as well.
Understanding the differences between RNASE A and H as nucleases that cut nucleic acids is integral for understanding their respective substrate specificities, roles in cell biology, and biological purposes – including studying the metabolism of both RNA and DNA as well as designing therapeutic interventions and antiviral drugs.
RNASEs and their biological significance
RNASEs, or ribonucleases, are enzymes that catalyze the degradation or modification of RNA molecules. They play crucial roles in various biological processes and have significant biological significance.
Here are some key points regarding RNASEs and their biological significance:
- RNA Degradation and Turnover: RNASEs are responsible for the degradation and turnover of RNA molecules within cells. They play a crucial role in maintaining proper RNA levels, controlling gene expression, and eliminating aberrant or unnecessary RNA molecules. This process ensures cellular homeostasis and regulation of biological processes.
- RNA Quality Control: RNASEs contribute to quality control mechanisms by recognizing and degrading faulty or damaged RNA molecules. They help maintain the integrity of the cellular RNA pool by eliminating RNA with errors or mutations.
- Cellular Defense: Some RNASEs are part of the innate immune response and act as a defense mechanism against foreign RNA, such as viral RNA. These RNASEs recognize and degrade the viral RNA, limiting viral replication and spread.
- Maturation of Functional RNAs: RNASEs are involved in the processing and maturation of functional RNAs. For example, during the maturation of transfer RNA (tRNA) molecules, specific RNASEs cleave and modify tRNA precursors, generating functional tRNA molecules necessary for protein synthesis.
- RNA Interference (RNAi): RNASEs play a critical role in RNA interference, a mechanism involved in gene regulation. Under RNAi, specific RNASEs such as Dicer are utilized to break long dsRNA into short interfering and microRNA molecules called siRNAs and miRNAs which act as guides for silencing complexes to target messenger RNAs for degradation or translational repression.
- Biotechnological and Therapeutic Applications: RNASEs have applications in various biotechnological and therapeutic fields. They are used in RNA purification, RNA structure analysis, RNA sequencing, and other techniques involving RNA manipulation. Additionally, some RNASEs are being explored as potential therapeutic agents for specific diseases, including cancer.
RNASEs are essential enzymes involved in RNA degradation, quality control, cellular defense, RNA maturation, and gene regulation. Their biological significance lies in maintaining RNA homeostasis, regulating gene expression, and participating in crucial cellular processes. Additionally, RNASEs have practical applications in biotechnology and hold therapeutic potential in specific contexts.
Importance of understanding the difference between RNASE A and RNASE H
Understanding the difference between RNASE A and RNASE H is of vital significance for several reasons, including cell processes:
- Cellular Processes: Both proteins play distinctive roles within different cellular functions. RNASE A is involved in RNA degradation and turnover, regulating gene expression, and host defense against foreign RNA. On the other hand, RNASE H is crucial for DNA replication, repair, and retroviral replication. Understanding their specific functions helps elucidate the mechanisms and regulation of these cellular processes.
- RNA-DNA Hybrids: RNASE A and RNASE H have different substrate specificities. While RNASE A cleaves RNA molecules, RNASE H specifically targets RNA-DNA hybrids. RNA-DNA hybrids are formed during DNA replication, repair, transcription, and retroviral replication. Differentiating between these two enzymes helps in understanding the specific roles of each enzyme in processing and resolving RNA-DNA hybrids.
- Therapeutic Applications: The knowledge of RNASE A and RNASE H differences has implications in therapeutic applications. For example, targeting RNASE H activity has been explored as a strategy to inhibit retroviral replication and develop antiviral drugs. Understanding the unique features and functions of RNASE H assists in designing effective therapeutic interventions against retroviral infections.
- Biotechnological Applications: RNASE A and RNASE H find applications in various biotechnological processes. RNASE A is used extensively in molecular biology and biotechnology research for applications including purifying RNA for analysis or sequencing purposes. RNASE H, with its ability to selectively degrade RNA in RNA-DNA hybrids, is utilized in techniques like reverse transcription and cDNA synthesis.
- Research and Knowledge Expansion: Studying the differences between RNASE A and RNASE H contributes to the broader understanding of nucleic acid metabolism and cellular processes. Enhancing our understanding of RNA and DNA biology, gene regulation and replication mechanisms (such as replication repair mechanisms ) plays a fundamental role in furthering scientific research in various disciplines including molecular biology, genetics and virology.
Comprehending the distinctions between RNASE A and RNASE H is crucial for understanding their specific functions, cellular roles, and implications in various biological processes. It aids in therapeutic developments, biotechnological applications, and the expansion of scientific knowledge in nucleic acid biology.
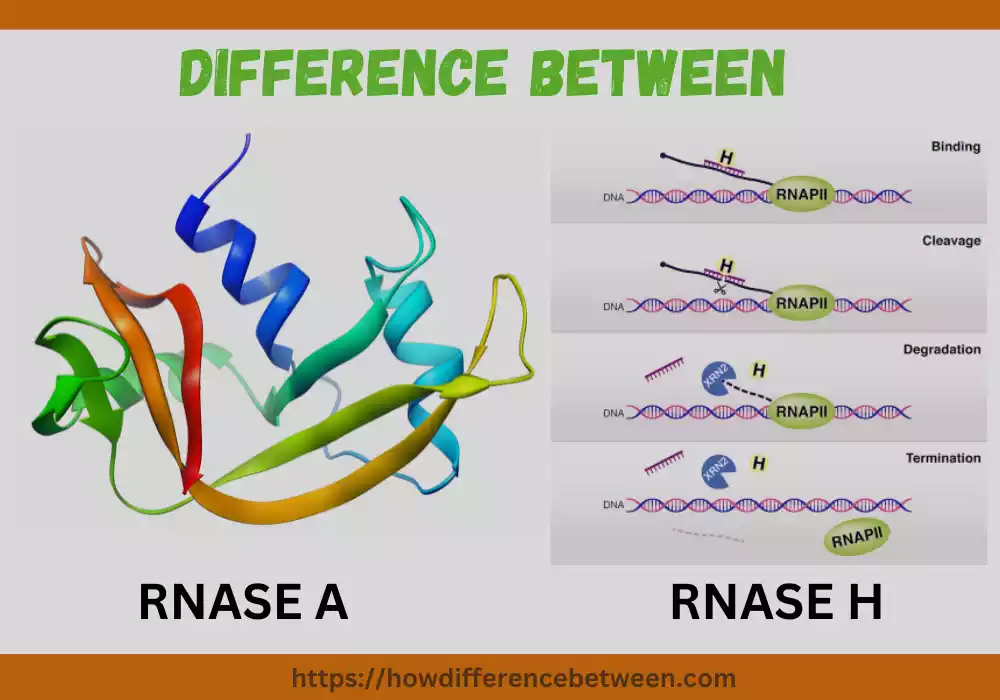
RNASE A
RNASE A is an endoribonuclease enzyme that specifically cleaves RNA molecules. Here is a more detailed overview of RNASE A:
- Structure and Composition:
- RNASE A is a small, single-chain protein enzyme.
- Composed of 124 amino acids and having an approximate molecular weight of 13.7kDa.
- The protein structure of RNASE A is stabilized by four disulfide bonds, which contribute to its stability and resistance to proteolysis.
- Function and Role in Cells:
- RNASE A is primarily known for its involvement in RNA degradation and turnover in cells.
- It recognizes specific sequences or structures in RNA molecules and cleaves the phosphodiester bonds, resulting in the fragmentation of RNA.
- By degrading RNA, RNASE A regulates RNA stability, controls gene expression, and eliminates aberrant or unnecessary RNA molecules.
- Additionally, RNASE A plays a role in host defense and immunity by degrading foreign RNA, such as viral RNA.
- Substrate Specificity and Cleavage Sites:
- RNASE A exhibits specificity towards RNA substrates, recognizing specific sequences or secondary structures.
- It cleaves RNA at the 3′ side of pyrimidine nucleotides (cytidine and uridine).
- The cleavage sites typically occur between the phosphate group of one nucleotide and the ribose of the adjacent nucleotide.
- Biological Functions:
- RNA Degradation and Turnover: RNASE A participates in the degradation and turnover of RNA molecules, ensuring proper regulation of gene expression and cellular homeostasis.
- Host Defense and Immunity: RNASE A helps defend against foreign RNA, including viral RNA, by degrading the RNA of invading pathogens and contributing to the immune response.
- Applications and Research:
- Biotechnological and Industrial Uses: RNASE A is widely utilized in molecular biology and biotechnology research. It is employed in RNA purification, RNA structure analysis, RNA sequencing, and other techniques involving RNA manipulation.
- Study of RNA Structure and Function: RNASE A has been instrumental in the study of RNA structure, folding, and function. Its ability to cleave RNA at specific sites aids in determining the secondary and tertiary structure of RNA molecules.
RNASE A is an essential enzyme in RNA metabolism, playing significant roles in regulating gene expression, RNA degradation, and host defense. Biochemically speaking, its biochemical properties and applications make RNA an invaluable research tool in various research fields; contributing greatly to our knowledge about its biology as well as potential health and disease consequences.
How does RNASE A degrade RNA molecules?
RNASE A degrades RNA molecules through an endoribonuclease activity, meaning it cleaves RNA at specific internal sites rather than at the ends.
Here’s an overview of how RNASE A degrades RNA:
- Recognition of RNA Substrate: RNASE A recognizes specific sequences or secondary structures in RNA molecules. The enzyme interacts with the target RNA through electrostatic interactions, hydrogen bonding, and hydrophobic interactions.
- Cleavage of Phosphodiester Bonds: Once bound to the RNA substrate, RNASE A catalyzes the hydrolysis of the phosphodiester bonds between adjacent ribonucleotides. It breaks the bonds by attacking the scissile phosphate group, leading to the fragmentation of the RNA molecule.
- Cleavage Preferences: RNASE A has a specific cleavage preference for RNA substrates. It cleaves RNA at the 3′ side of pyrimidine nucleotides, specifically cytidine (C) and uridine (U). The cleavage sites typically occur between the phosphate group of one nucleotide and the ribose of the adjacent nucleotide.
- Multiple Cleavage Events: RNASE A can cleave RNA at multiple sites along the molecule, resulting in the generation of smaller RNA fragments. The process continues until the RNA molecule is extensively degraded or the enzyme dissociates from the substrate.
- Release of Cleavage Products: As RNASE A cleaves the RNA molecule, it releases smaller RNA fragments or oligonucleotides as degradation products. These fragments can be further processed or utilized by the cell for various purposes.
It’s important to note that the specific cleavage sites and efficiency of RNA degradation by RNASE A can be influenced by factors such as the RNA sequence, secondary structure, and the presence of other proteins or cofactors.
RNASE A degrades RNA molecules by cleaving the phosphodiester bonds within the RNA chain, leading to the fragmentation and degradation of the RNA substrate. Its endoribonuclease activity and specific cleavage preferences contribute to its role in RNA degradation, turnover, and regulation of gene expression.
RNASE H
RNASE H is an endonuclease enzyme that specifically cleaves RNA-DNA hybrids.
Here is an in-depth review of RNASE H:
- Structure and Composition:
- RNASE H is found both prokaryotic cells as well as eukaryotic organisms..
- It can exist in multiple isoforms with varying sizes and subunit compositions.
- The enzyme typically consists of a catalytic domain responsible for the cleavage activity and may have additional auxiliary domains.
- Function and Role in Cells:
- RNASE H is primarily involved in the processing and removal of RNA primers during DNA replication and repair.
- It recognizes and specifically cleaves the RNA strand within an RNA-DNA hybrid structure.
- The cleavage by RNASE H allows DNA polymerases or repair enzymes to fill in the resulting gap with DNA.
- Substrate Specificity and Cleavage Sites:
- RNASE H exhibits specificity towards RNA-DNA hybrids, which are formed during various cellular processes such as DNA replication, DNA repair, and transcription.
- It recognizes the RNA strand within the hybrid and catalyzes the cleavage of the RNA strand.
- The cleavage sites typically occur at the RNA-DNA junction, resulting in the removal of the RNA component.
- Biological Functions:
- DNA Replication and Repair: RNASE H is critical for the removal of RNA primers during DNA replication. It ensures the synthesis of uninterrupted DNA strands and facilitates the subsequent joining of DNA fragments during Okazaki fragment maturation.
- Retroviral Replication: RNASE H plays a vital role in retroviral replication. Retroviruses use their RNA genomes as templates for reverse transcription, resulting in the formation of RNA-DNA hybrids. RNASE H degrades the RNA strand within the hybrid, allowing the synthesis of the complementary DNA strand.
- Applications and Research:
- Study of DNA-RNA Hybrids: RNASE H is extensively used in research to study and analyze RNA-DNA hybrid structures. Its ability to specifically cleave the RNA component aids in the investigation of hybrid formation, stability, and dynamics.
- Antiviral Drug Development: Inhibiting RNASE H activity has been explored as a potential therapeutic strategy against retroviral infections. Developing compounds that selectively target RNASE H could disrupt viral replication and hinder the progression of viral diseases.
RNASE H plays a crucial role in DNA replication, repair, and retroviral replication by specifically cleaving RNA-DNA hybrids. Its activity is essential for maintaining genomic integrity and proper functioning of cellular processes. Understanding the specific functions and mechanisms of RNASE H contributes to advancements in fields such as DNA metabolism, antiviral drug development, and the study of RNA-DNA interactions.
What is the role of RNASE H in DNA replication and repair?
RNASE H plays a critical role in DNA replication and repair by removing RNA primers that are involved in the initiation of DNA synthesis.
Here’s an overview of the role of RNASE H in DNA replication and repair:
- DNA Replication: During DNA replication, both leading and lagging strands are synthesized in a discontinuous manner. On the lagging strand, RNA primers are initially synthesized by the primase enzyme. These RNA primers provide a starting point for DNA synthesis by DNA polymerase.
- RNA Primer Removal: Once the RNA primers have fulfilled their role in DNA synthesis, they need to be removed to generate a continuous DNA strand. This is where RNASE H comes into play. RNASE H specifically recognizes and cleaves the RNA component of RNA-DNA hybrids, which are formed between the RNA primer and the newly synthesized DNA.
- Gap Filling: After the RNA primer is removed by RNASE H, the resulting gap is filled in by DNA polymerase using the available 3′-OH end of the upstream DNA fragment as a primer. The newly synthesized DNA is then ligated to the adjacent DNA fragment, resulting in a continuous DNA strand.
- Okazaki Fragment Maturation: On the lagging strand, DNA synthesis occurs in short fragments called Okazaki fragments. RNASE H’s role in removing the RNA primers allows for the joining of adjacent Okazaki fragments during their maturation. Once the RNA primers are removed and the gaps are filled, DNA ligase seals the nicks, resulting in a fully replicated and continuous DNA strand.
- DNA Repair: RNASE H also participates in DNA repair processes that involve the removal of RNA primers. Various DNA repair mechanisms, such as base excision repair and nucleotide excision repair, involve the removal of damaged DNA and subsequent DNA synthesis. RNASE H aids in the removal of RNA primers during these repair processes to facilitate DNA repair and restoration of genomic integrity.
RNASE H plays a crucial role in DNA replication by removing RNA primers, allowing for the synthesis and joining of DNA fragments. It is involved in the maturation of Okazaki fragments on the lagging strand. RNASE H participates in DNA repair mechanisms that involve the removal of RNA primers during the repair of damaged DNA.
Comparison table of RNASE A and RNASE H
Here’s a comparison chart highlighting the key differences between RNASE A and RNASE H:
| Aspect | RNASE A | RNASE H |
|---|---|---|
| Structure | Single-chain protein enzyme | Multiple isoforms with varying sizes and subunit compositions |
| Substrate specificity | Cleaves RNA molecules | Cleaves RNA-DNA hybrids |
| Cleavage preference | Cleaves RNA at the 3′ side of pyrimidine nucleotides | Cleaves the RNA strand within RNA-DNA hybrids |
| Biological functions | RNA degradation and turnover, gene expression regulation, host defense against foreign RNA | Removal of RNA primers during DNA replication and repair, involvement in retroviral replication |
| Usage in research | RNA purification, RNA structure analysis, RNA sequencing | Study of DNA-RNA hybrids, investigation of retroviral replication, potential target for antiviral drug development |
Similarities Between RNASE A and RNASE H
RNASE A is a different enzyme with a distinct function, but there are some similarities between the two:
- Enzymatic Activity: RNASE A as well as RNASE H catalyze nucleic acid Cleavage. They differ in the specificity of their substrates, with RNASE targeting RNA molecules, and RNASE cleaving RNA/DNA hybrids.
- Catalytic Mechanism: Both enzymes use a similar catalytic system involving hydrolysis of the phosphodiester bond. They break the bond between adjacent nucleotides leading to fragmentation of nucleic acids substrate.
- Biological Significance: RNASE A as well as RNASE H are both of significant biological importance in cellular processes. They are involved in maintaining genomic integrity, regulating genes expression and nucleic acid metabolic processes.
- Research Applications: Both enzymes have been widely used in research on molecular biology, biotechnology and genetics. RNASE A can be used for RNA purification and analysis. RNASE H can be used to study DNA-RNA hybrids, as well as processes like DNA replication, DNA repair, and retroviral reproduction.
While RNASE A & RNASE H have some similarities, their functions and specificities are different. Understanding their differences will help you study their roles and conduct research in the relevant fields.
How do RNASE A and RNASE H differ in terms of structure and function?
RNASE A and RNASE H differ in terms of their structure and function. Here’s a comparison between the two:
Structure:
- RNASE A: RNASE A is a single-chain protein enzyme composed of 124 amino acids. It adopts a compact globular structure stabilized by disulfide bonds. The enzyme has a well-defined active site that allows it to interact with and cleave RNA molecules.
- RNASE H: RNASE H can exist in multiple isoforms with varying sizes and subunit compositions. It typically consists of a catalytic domain responsible for the cleavage activity and may have additional auxiliary domains. The structure of RNASE H may vary depending on the organism and isoform.
Function:
- RNASE A: RNASE A is a ribonuclease that specifically cleaves RNA molecules. It recognizes and binds to RNA substrates, cleaving them at specific sites. RNASE A is involved in RNA degradation and turnover, gene expression regulation, and defense against foreign RNA.
- RNASE H: RNASE H is an endonuclease that specifically cleaves RNA-DNA hybrids. It recognizes the RNA component within the hybrid and catalyzes the cleavage of the RNA strand. RNASE H plays a crucial role in DNA replication and repair by removing RNA primers, facilitating gap filling, and promoting the maturation of Okazaki fragments. It is also involved in retroviral replication by degrading the RNA strand of RNA-DNA hybrids during reverse transcription.
Substrate Specificity:
- RNASE A: RNASE A exhibits specificity towards RNA substrates and cleaves RNA molecules. It recognizes specific sequences or secondary structures in RNA molecules and cleaves RNA at the 3′ side of pyrimidine nucleotides (cytidine and uridine).
- RNASE H: RNASE H exhibits specificity towards RNA-DNA hybrids and cleaves the RNA component within the hybrid. It recognizes the RNA-DNA junction and catalyzes the cleavage of the RNA strand.
While both RNASE A and RNASE H are nucleases involved in nucleic acid processing, they differ in terms of their specific functions, substrate specificity, and structural characteristics. RNASE A primarily acts on RNA molecules, while RNASE H targets RNA-DNA hybrids. Understanding these differences is crucial for studying their individual roles and their significance in cellular processes.
What are the potential future research areas for RNASE A and RNASE H?
Potential future research areas for RNASE A and RNASE H can include:
RNASE A:
- RNA Structure and Function: Further exploration of the structural properties and functional roles of RNA molecules and their interactions with RNASE A. This can involve studying RNA folding, RNA-protein interactions, and RNA-based regulatory mechanisms.
- Therapeutic Applications: Investigating the potential therapeutic applications of RNASE A or its derivatives. This can involve developing RNASE A-based therapies for diseases associated with aberrant RNA metabolism, such as neurodegenerative disorders, viral infections, or cancer.
- Engineering and Modification: Exploring the possibilities of engineering or modifying RNASE A to enhance its catalytic activity, substrate specificity, or stability. This can lead to the development of improved RNASE A variants with enhanced properties for various applications.
RNASE H:
- Mechanisms of RNA-DNA Hybrid Recognition: Investigating the mechanisms by which RNASE H recognizes and cleaves RNA-DNA hybrids. Understanding the structural and molecular basis of RNA-DNA hybrid recognition can provide insights into the regulation of DNA replication, repair, and retroviral replication.
- Interplay with DNA Replication and Repair Machinery: Exploring the interactions and coordination of RNASE H with other proteins and enzymes involved in DNA replication and repair. This can include studying the dynamics of RNASE H activity during different stages of DNA replication and repair processes.
- Therapeutic Strategies: Examining the potential therapeutic strategies targeting RNASE H for specific diseases. This can involve developing small molecule inhibitors or RNA-based approaches to modulate RNASE H activity and influence DNA replication, repair, or viral replication processes.
- Structural and Functional Variants: Investigating the structural and functional diversity of RNASE H isoforms across different organisms and cell types. This can provide insights into the functional specialization and evolutionary aspects of RNASE H enzymes.
Future research on RNASE A and RNASE H can focus on expanding our understanding of their roles in RNA and DNA metabolism, exploring their therapeutic potential, uncovering the mechanisms underlying their activities, and discovering new variants or modifications with enhanced properties for various applications.
Future Directions and Potential Research Areas
Future research directions and potential areas of study for RNASE A and RNASE H can include:
- Mechanistic Insights: Investigating the detailed mechanisms of action and substrate recognition for RNASE A and RNASE H. This can involve structural studies, mutagenesis experiments, and biochemical assays to elucidate the precise molecular interactions and catalytic mechanisms of these enzymes.
- Substrate Specificity: Exploring the factors influencing substrate specificity for both RNASE A and RNASE H. This can include studying the sequence and structural determinants that dictate substrate recognition and cleavage preferences, as well as identifying any potential cofactors or interacting proteins involved in modulating their activities.
- Functional Interactions: Investigating the functional interactions and crosstalk between RNASE A and RNASE H with other nucleases, RNA-binding proteins, and DNA repair factors. Understanding how these enzymes coordinate their activities within the context of complex cellular processes can provide valuable insights into their functional roles and regulatory mechanisms.
- Disease Relevance: Exploring the relevance of RNASE A and RNASE H in disease contexts. This can involve studying their dysregulation or altered expression patterns in various diseases, such as cancer, neurodegenerative disorders, or viral infections. Additionally, investigating the potential therapeutic targeting of these enzymes for disease treatment or intervention.
- Novel Functions and Substrates: Searching for novel functions and substrates of RNASE A and RNASE H beyond their established roles. This can involve screening approaches, high-throughput sequencing technologies, and omics-based analyses to identify new RNA or DNA targets that may be subject to their enzymatic activities.
- Engineering and Manipulation: Exploring the possibilities of engineering RNASE A and RNASE H for improved functionalities, substrate specificities, or altered enzymatic properties. This can involve protein engineering techniques, directed evolution, or rational design to generate variants with enhanced characteristics for specific applications.
- Therapeutic Applications: Investigating the therapeutic potential of RNASE A and RNASE H as targets for drug development or therapeutic interventions. This can include developing small molecules, peptides, or RNA-based approaches to modulate their activities for the treatment of diseases associated with RNA or DNA dysregulation.
These research directions can contribute to a deeper understanding of the biological functions, regulatory mechanisms, and potential applications of RNASE A and RNASE H in various fields, including molecular biology, genetics, biotechnology, and medicine.
Summary
RNASE A and RNASE H are indispensable players in the intricate world of RNA metabolism. RNASE A’s ability to degrade single-stranded RNA molecules makes it a versatile tool in molecular biology and potential cancer therapy.
RNASE H’s role in RNA-DNA hybrid degradation holds promise for understanding genetic disorders and immune responses. Unlocking the secrets of these enzymes paves the way for groundbreaking discoveries in biology and medicine.