Introduction to Heat Transfer and Thermodynamics
Heat Transfer and Thermodynamics are essential concepts in the field of energy and engineering. Heat transfer focuses on the transfer of thermal energy between objects or regions through conduction, convection, and radiation. Thermodynamics, on the other hand, studies the principles and laws governing energy and its transformations in systems.
While both concepts are interrelated, they differ in scope, analytical tools, and applications. Understanding the differences between heat transfer and thermodynamics is crucial for designing efficient energy systems and solving real-world engineering challenges.
Importance and Applications of Heat Transfer and Thermodynamics Concepts
- Energy Efficiency: Understanding heat transfer processes is integral for designing energy-efficient systems. Optimizing thermal insulation or heat exchanger processes, for instance, can significantly lower energy use while leading to cost savings and reduced environmental impacts.
- HVAC Systems: Heat transfer principles play a crucial role in designing and operating HVAC systems. Proper heat transfer ensures efficient heating and cooling in buildings for greater comfort as well as better indoor air quality control.
- Industrial Processes: Many industrial processes involve heat transfer, such as chemical reactors, furnaces, and boilers. Efficient heat transfer enables optimal process control, productivity and safety in these facilities.
- Thermal Management: Heat transfer plays an essential part in dissipating heat in electronic devices, power systems, and automotive engines, helping ensure optimal performance, reliability and lifespan of these systems. Effective thermal management enhances these attributes as well.
- Renewable Energy: Heat transfer principles play an integral part of renewable energy technologies like solar thermal systems and geothermal heat pumps, making an understanding of heat transfer essential to effectively harnessing renewable sources of energy into usable forms.
Definition of Heat
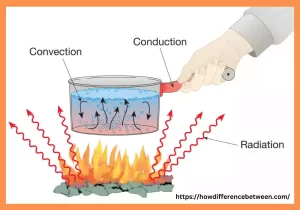
Heat transfer refers to energy transfer between objects due to differences in temperature. It is an essential process that happens in nature and plays a vital function in many engineering and daily applications. The process of heat transfer occurs by way of three different ways including conduction, convection, and radiation. Conduction is when heat is transferred by direct contact between two objects or within a solid substance.
Convection is the process of transferring heat by the motion of fluids or gases. Radiation is caused by electromagnetic waves, and doesn’t require a medium to facilitate heat transfer. Understanding how heat is transferred is vital to develop efficient cooling and heating systems as well as thermal management for manufacturing processes and for optimizing energy use.
Theory of Heat Transfer
Heat transfer theory refers to a set of principles and mathematical models used to understand, model, predict and quantify thermal energy transfer between objects or systems. Prediction and quantification allow us to accurately forecast heat transfer rate as well as its subsequent temperature distribution patterns.
Heat transfer theory relies on three primary forms of transference – conduction, convection and radiation. Each form possesses its own set of principles and mathematical equations for operation.
- Conduction: Conduction refers to the transmission of heat inside a solid or between solids when in direct physical contact. Conduction occurs by the transference of energy between higher-energy particles to lower-energy particles in a substance, known as conduction. Its rate depends on several variables including variations in temperature as well as thermal conductivity of materials used and dimensions across which heat travels across cross sections.
Fourier’s Law of Conduction serves as the basic equation used to explain this phenomenon. It can be described in Q = -kA(dT/dx) in which Q is the heat transfer rate, k represents temperature conductivity and A represents the area that is cross-sectional that heat flows through, and (dT/dx) represents the heat gradient.
- Convection: Convection refers to the process of transferring heat through the movement of fluids (liquids or gasses). Convection occurs because of the combined effect of conduction and fluid movement. Convection is divided into two kinds that are natural and forced convection. Natural convection is caused by the differences in density caused by fluctuations in temperature, which trigger fluid movement.
Convection that is forced involves the use of external devices like pumps or fans, to increase fluid movement and transfer of heat. Convective heat transfer rates depend upon many variables including surface area, temperatures, and fluid properties as well as the coefficient of convective heat transfer coefficient.
- Radiation: Radiation is the transmission of heat by electromagnetic waves. Contrary to convection and conduction, radiation is able to occur even in a vacuum because it doesn’t require any medium to propagate. Every object that has an absolute temperature radiates heat and absorbs it. Based on the Stefan-Boltzmann Law, the radiative heat transfer speed will be proportional to the absolute temperature differential between the two surfaces.
What are modes of heat transfer?
There are three primary modes of heat transfer:
- Conduction: Conduction is a method of heat transfer that occurs via direct contact between the particles of the same substance, or between various materials in physical contact. In solids, heat gets transferred through the vibrating of free electrons or atoms that transfer energy between particles to the following. Good conductors, for instance metals, permit heat to move more quickly in a more efficient manner, while insulators like plastic or wood, hinder the circulation of heat.
- Convection: Convection is a technic of transferring heat that takes place through the movement of liquids. It is the process of transferring heat through the motion of the fluid. Convection is further divided into two kinds that are natural and forced convection.
- Radiation: Radiation refers to the process of transferring heat which occurs via electromagnetic waves without the requirement of any medium and direct contact. Contrary to conduction and convection, radiation is possible in a vacuum. Anything above absolute temperature radiate and absorb radiation. The amount of radiation transferred depends on the temperature as well as the emissivity of the materials affected. The rougher and darker surfaces are likely to have better absorption and radiators.
Definition of Thermodynamics

Thermodynamics is a branch of physics concerned with energy, its transformations, and how heat converts into work. This field seeks to understand and quantify system behavior with regard to flow of energy in each system and evaluate any flows or reserves within them. Thermodynamics examines properties and states of matter while developing principles governing their behavior as energy flows within an economy or country.
Thermodynamic systems can be defined as any region or object under consideration, while their surroundings encompass everything outside this particular system. The discipline of thermodynamics relies upon four fundamental laws of action.
- The Zeroth Law of Thermodynamics states: If two systems are in thermal equilibrium with each other and with any third system, they are also thermally equivalent.
- The First Law of Thermodynamics (Law of Energy Conservation) states: An isolated system’s total energy remains constant over time; only its form or transfer between systems can change.
- The Second Law of Thermodynamics: The Second Law of Thermodynamics holds that, over time, an isolated system tends to gain in entropy; meaning natural processes tend toward increased disorder or randomness (increased entropy).
- The Third Law of Thermodynamics states: As temperature approaches absolute zero, its entropy decreases until reaching its minimum value.
Thermodynamics encompasses the study of thermodynamic processes and cycles, such as isothermal, adiabatic, isobaric, and isochoric processes. Additionally, thermodynamics involves understanding key thermodynamic properties like internal energy, enthalpy, entropy, and specific heat capacities as well as using equations of state like the ideal gas law to represent relationships among pressure, volume, and temperature.
Thermodynamics has applications across numerous fields, such as engineering, physics, chemistry and environmental science. It serves as the cornerstone for understanding energy systems such as power generation, refrigeration engines and heat transfer processes, and understanding thermodynamics can enable scientists and engineers to make educated decisions regarding energy efficiency, resource utilization and system design.
Theory of Thermodynamics
The concept of thermodynamics is built on a set of fundamental laws and principles which govern the behavior of energy in diverse ways.
The laws and principles are:
- Zeroth Laws of Thermodynamics: This law defines the idea of temperature as well as thermal equilibrium. It says that when two systems are separated by thermal equilibrium and another system, they’re in equilibrium. This allows the determination of temperature scales as well as the evaluation of the thermal state.
- First Law of Thermodynamics (Conservation of Energy): The law is based upon the idea of energy conservation. The law states that energy cannot be manufactured or destroyed, it is able to be moved into various types (such in the form of heat or work). Mathematically speaking, it is stated in terms of DU = Q + W. In this equation, DU represents the alteration in energy within this system. The Q refers to adding heat to the system and W represents the work that the system performs.
- Second Law of Thermodynamics: The law states that the total amount of entropy in an isolated system will always increase over time or is constant during irreversible phenomena. This also serves as the foundation for the classification of the efficiency of heat engines, as well as their limit.
- The Third Law of Thermodynamics: This law is related to the nature of systems when they are at zero temperatures (0 Kelvin). As the temperature of a system begins to reach the absolute point, its energy will be at the minimum value or is constant. This implies that reaching the temperature of absolute zero isn’t achievable.
The thermodynamic theory also includes a range of thermodynamic processes as well as cycles such as isothermal, adiabatic, and isochoric cycles. It is the study of the properties of pressure, volume as well as temperature as well as entropy. These properties are correlated with respect to the system. Thermodynamic equations, like that of the perfect gas law as well as particular heat capacities, are utilized to analyze and explain the behavior of substances and materials.
Importance and Applications of Thermodynamics
- Energy Conversion: Thermodynamics plays an essential role in studying energy conversion processes such as power production by steam turbines, internal combustion engines and renewable energy systems. It helps optimize their efficiency and performance to help maximize efficiency and performance for these systems.
- Refrigeration and Air Conditioning: Thermodynamics provides the foundation of refrigeration and air conditioning systems, enabling heat transfer between lower temperature regions to higher temperature regions for cooling comfort.
- Chemical Reactions and Processes: Thermodynamics is used to analyze and optimize chemical reactions and processes. It determines their feasibility, equilibrium conditions and efficiency while helping inform reactor design decisions.
- Environmental Systems: Thermodynamics is an integral component of environmental engineering and sustainability, helping engineers evaluate energy flows, pollution controls and human activities’ effect on ecosystems.
- Material Science: Thermodynamics is central to understanding phase transitions such as solidification, melting, and vaporization and provides insights into material properties, stability and behavior under various environmental conditions.
- Aerospace and Automotive Engineering: Thermodynamics is essential in designing propulsion systems like jet engines and rockets to increase fuel efficiency while improving performance. It helps optimize both designs.
- Biological Systems: Thermodynamics can be utilized for studying biological systems such as metabolism and bioenergetics to gain greater insight into energy transfer within living organisms and its transformation processes.
What are the 4 laws of thermodynamics?
The thermodynamics laws, which are four in number are the fundamental laws that regulate energy behavior and systems. They serve as a foundation that allows for the understanding and analysis of the thermodynamic process.
There are the main Four laws:
- Zeroth Law of Thermodynamics: The Zeroth Law establishes the idea of temperature and equilibrium. The law states that if two systems exist independently in equilibrium with another system, they are at thermal equilibrium with one another. This allows the identification of temperature scales, as well as the evaluation of the thermal state.
- First Law of Thermodynamics (Conservation of energy): This first law in thermodynamics is based on the idea that energy
protection is a significant component. It holds that energy can’t be destroyed or created, it may transfer between various kinds of forms (such in the form of heat or work). It is the First Law is often expressed in the form of a formula: D = Q – W. In this equation, DU refers to the energy change within of a system. Q represents the amount of heat that is it adds to the system while W refers to what is done by the system. - The Second law of Thermodynamics: This law provides the concept of entropy. It also helps explain the origins of natural phenomena. The law says that the total amount of entropy in an isolated system increases with time or is the same in processes that are reversible. This implies that some procedures are inherently irreversible, and the flow of heat is not spontaneous between a colder and the hotter body. This Second Law also provides the foundation for the classification of heat engines as well as their limits of efficiency.
- The Third Law of Thermodynamics: third law of Thermodynamics refers to the behavior of systems when they reach absolute zero temperatures (0 Kelvin). As the system reaches absolute zero, its entropy will be at an absolute value or a constant. This means that there is no way to attain absolute zero temperatures through an infinite number of steps. Third Law Third Law has implications for the behaviour of matter at extremely low temperatures as well as the physical properties of systems that occur in extreme temperatures.
The Key difference between Heat Transfer and Thermodynamics
- Scope: Heat transfer involves the exchange of thermal energy among objects or regions while thermodynamics deals with studying energy transformation in systems.
- Heat Transfer/Thermodynamics/Leveraging Systems: Heat transfer investigates the mechanisms and processes involved with transferring energy through conduction, convection and radiation; thermodynamics investigates principles governing energy transformation processes within systems.
- Analytical Tools: Heat transfer analysis typically employs mathematical equations and models specific to each mode of heat transfer, such as Fourier’s law for conduction or Newton’s cooling law in convection. Meanwhile, thermodynamics utilizes more general tools, including equations of state, thermodynamic laws (such as energy conservation or entropy laws), internal energy, enthalpy, or entropy as analytical concepts; etc.
- Time Dependency: Heat transfer studies the movement of energy between objects or regions at certain moments in time and thermodynamics examines its overall behavior and equilibrium states; thermodynamics studies this aspect only when considering all initial and final states as well as any intermediate states during processes.
- System Considerations: Heat transfer analysis tends to focus on individual objects or regions as energy transfers between them; this practice does not address overall system properties. By contrast, thermodynamics studies the behavior and properties of entire systems as a whole.
- Applications Focus: Heat transfer can be used both practically in engineering design applications as well as everyday systems to increase thermal exchange efficiency, while thermodynamics is more focused on understanding fundamental principles governing energy transformations which may apply across many fields including engineering, physics, chemistry, and environmental sciences.
Similarities of Heat Transfer and Thermodynamics
- Energy Transfer: Both heat transfer and thermodynamics examine how energy moves between objects; in terms of thermal transfer between two objects or between systems as wholes respectively, while thermodynamics investigates more general principles related to transference such as work energy exchange among systems as a whole and between sources within themselves.
- Conserve Energy: Both heat transfer and thermodynamics rely upon energy conservation as their cornerstone principle, with heat transfer often using the first law of thermodynamics (conservation of energy) as its basis to analyze and quantify heat transfers or work performed on systems. This ensures total system energy remains constant regardless of any heat transfers or work performed; accounting for any energy transfers or work completed by all components involved.
- Interdependency: Heat transfer is an integral component of thermodynamics, playing an indispensable part in energy transfer and system behavior. Thermodynamics outlines laws and principles governing heat transfer phenomena – understanding this data is vital in accurately analyzing thermodynamic processes or systems.
- Mathematical Modeling: Both heat transfer and thermodynamics utilize mathematical modeling as a means to study energy transfer and system behavior, using differential equations or mathematical relations as means for deriving equations that describe any related processes that arise during experiments or operations. This provides for easier analysis.
- Engineering Applications of Heat Transfer and Thermodynamics: Engineers have found numerous uses for both fields within engineering. Heat transfer principles play a pivotal role in designing efficient heat exchangers, HVAC systems and insulation materials while thermodynamics reveals insights into power generation systems, refrigeration units, and combustion engines. Combining both fields gives engineers a holistic view of energy transfer and system performance within various engineering applications.
Consultation
Heat transfer and Thermodynamics are fundamental concepts that govern energy exchange and the behavior of physical systems. From everyday applications to cutting-edge technologies, these principles shape the world around us. Understanding these principles is crucial for addressing environmental challenges and creating a sustainable future.